A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-DUAL NATURE OF RADIATION AND MATTER-Exercise
- lambda(e),lambda(p) and lambda(alpha) are the de-Broglie wavelength of...
Text Solution
|
- The ratio of the de-Broglie wavelengths of an electron of energy 10 eV...
Text Solution
|
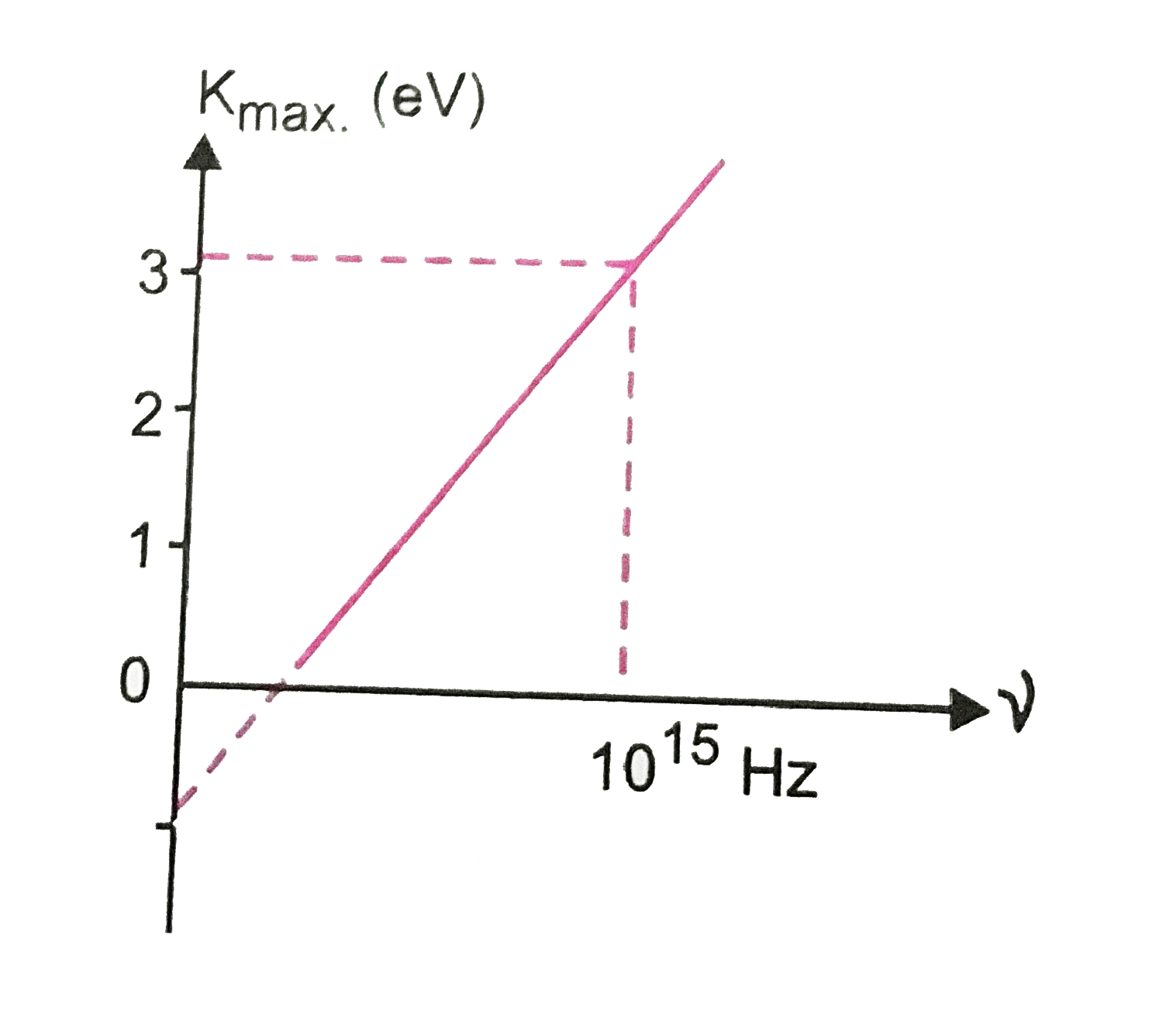
- represents a graph of most energetic photoelectrons K(max)(in eV) and ...
Text Solution
|
- Electrons used in an electron microscope are accelerated by a voltage ...
Text Solution
|
- After absorbing a slowly moving neutrons of mass m(N) (momentum ~0) a...
Text Solution
|
- A alpha -parhticle moves in a circular path of radius 0.83 cm in th...
Text Solution
|
- If the momentum of an electron is changed by p, then the de - Broglie ...
Text Solution
|
- An electron of mass m and a photon have same energy E. The ratio of de...
Text Solution
|
- Light of wavelength lambda(ph)falls on a cathode plate inside a vacuum...
Text Solution
|
- For photo - electric effect with incident photon wavelength lambda the...
Text Solution
|
- The maximum kinetic energy of the emitted photoelectrons against frequ...
Text Solution
|
- If the wavelength of light in an experiment on photoelectric effect is...
Text Solution
|
- Choose the incorrect statement:
Text Solution
|
- Photoelectric effect supports quantum nature of light because
Text Solution
|
- The maximum K.E. of photoelectrons ejected from a photometer when it i...
Text Solution
|
- An electron and proton have same de-Broglie wavelength. Which one poss...
Text Solution
|
- The graph between 1//lambda and stopping potential (V) of three metals...
Text Solution
|
- When a monochromatic point source of light is at a distance of 0.2 m f...
Text Solution
|
- Which of the following charaterstics of photoelectric effect supports ...
Text Solution
|
- The frequency and intensity of a light source are both doubled. Consid...
Text Solution
|