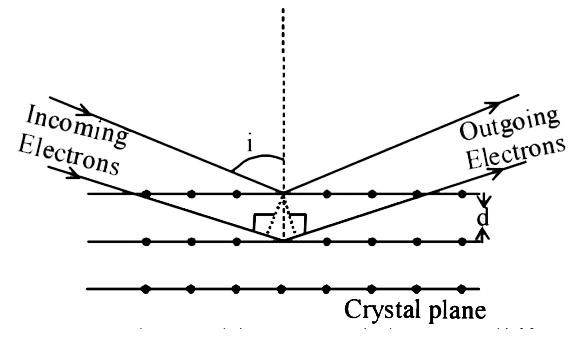
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-DUAL NATURE OF RADIATION AND MATTER-Exercise
- In which of the following cases the heavier of the two particles has a...
Text Solution
|
- Wave property of electron implies that they will show diffraction effe...
Text Solution
|
- Wave property of electron implies that they will show diffraction effe...
Text Solution
|
- Wave property of electron implies that they will show diffraction effe...
Text Solution
|
- In an experiment, electrons are made to pass through a narrow slit of ...
Text Solution
|
- A silver of radius 1 cm and work function 4.7 eV is suspended from an ...
Text Solution
|
- When a certain metallic surface is illuminated with monochromatic ligh...
Text Solution
|
- The work function of Silver and sodium are 4.6 and 2.3 eV, respective...
Text Solution
|
- A 25 watt bulb, which is producing monochromatic light of wavelength 6...
Text Solution
|
- A parallel beam of monochromatic light of wavelength 600nm is incident...
Text Solution
|
- A proton is fired from very far away towards a nucleus with charge Q ...
Text Solution
|
- De-broglie wavelength associated with an electron accelerated through ...
Text Solution
|
- Find the energy that should be added to an electron of energy 2eV to r...
Text Solution
|
- Assertion: Light of frequency 1.5 times the threshold frequency is inc...
Text Solution
|
- Assertion : Photoelectric effect demonstrates the wave nature of light...
Text Solution
|
- Assertion: Photoecell is called electric eye. Reason: Photocell can ...
Text Solution
|
- Assertion: An electron microscop can achieve better resolving power th...
Text Solution
|
- Assertiom: The de-broglie wavelength of a neutrons when its kinetic en...
Text Solution
|
- Assertion: The de-broglie wavelength equation has significance for any...
Text Solution
|
- Assertion: A particle of mass M at rest decays into two particles of m...
Text Solution
|