In the nth orbit of hydrogen atom, energy of electron, `E_(n)=(-13.6)/(n^(2))eV`
In case of hydrogen like atom, the energy is `E_(n)'=3^(2)(E_(n))=(-9xx13.6)/(n^(2))eV`
for n=1, `E_(1)'=-9xx13.6eV`,
for n=3, `E_(3)'=(-9xx13.6)/(3^(2))=-13.6 eV`
Energy of photon required to excite the electron form n=1 to n=3 is
`hv=E_(3)'-E_(1)'=-13.6-(-9xx13.6)=8xx13.6eV`
`(hc)/lambda=8xx13.6xx1.6xx10^(-19)("joule")`
`lambda=(hc)/(8xx13.6xx1.6xx10^(-19))=(6.63xx10^(-34)xx3xx10^(8))/(8xx13.6xx1.6xx10^(-19))=114.25xx10^(-10)m=114.25Å`
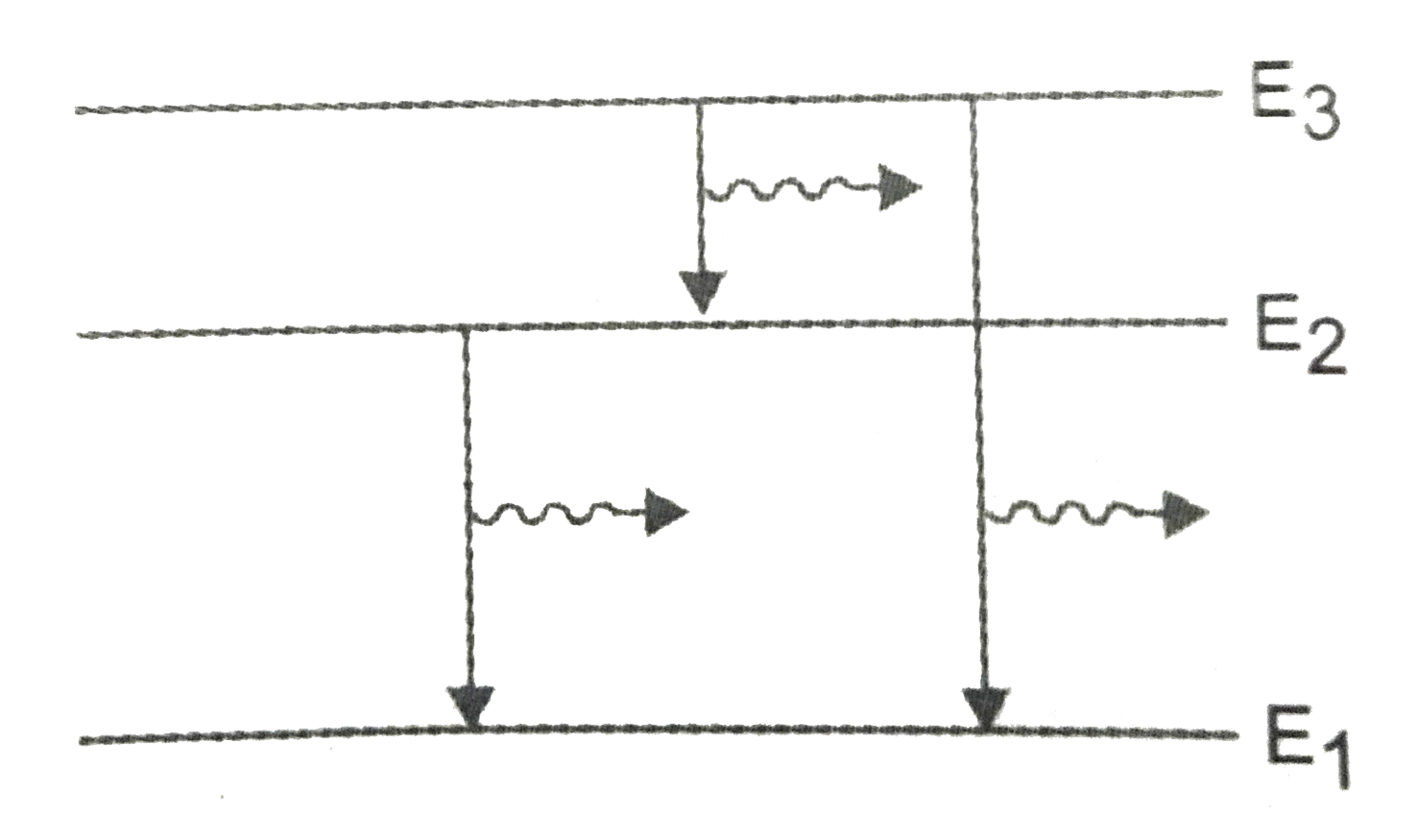
This is the wavelength of the radiation requirede. The possible spectral lines observed in this system are three : `E_(3)toE_(1),E_(3)toE_(2) and E_(2)toE_(1)` as shown in fig.