Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-ATOMS AND NUCLEI-Exercise
- A hydrogen atom initially in the ground level absorbs a photon, Which ...
Text Solution
|
- A 12.9 eV beam of electrons is used to bombard gaseous hydrogen atom a...
Text Solution
|
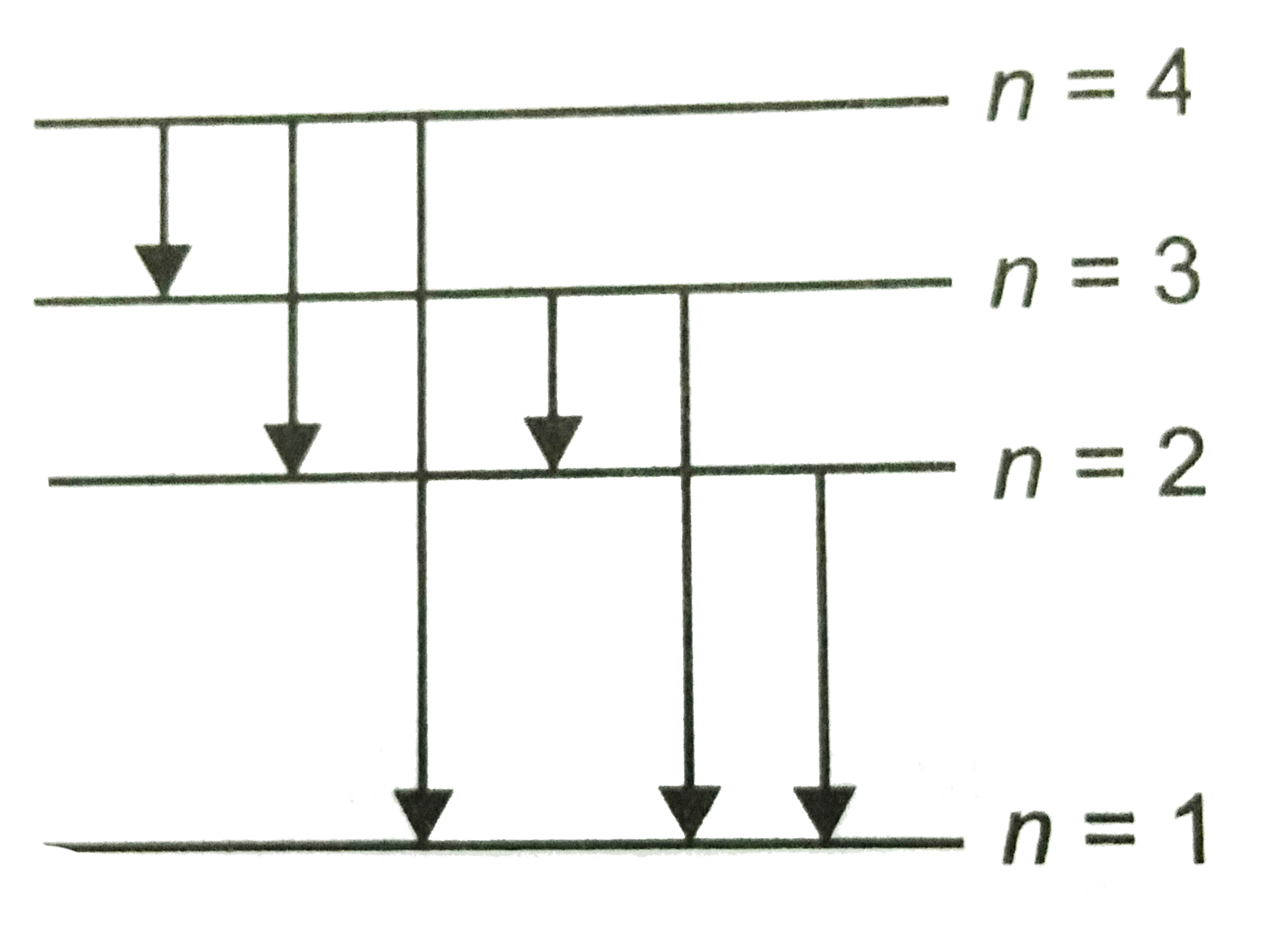
- Fig. shows energy level diagram of hydrogen atom. Find out the transit...
Text Solution
|
- A hydrogen atom rises form its n=1 state to the n=4 state by absorbing...
Text Solution
|
- If in nature they may not be an element for which the principle quantu...
Text Solution
|
- Which state of the triply ionized Be^(+++) has the same orbital radius...
Text Solution
|
- What is the relation between decay constant and half life of a radio a...
Text Solution
|
- 1curei=k disintegrations/sec, where k is
Text Solution
|
- Which of the following is in the increasing order for penetrating powe...
Text Solution
|
- Two nuclei have mass number in the ratio 1:8. What is the ratio of the...
Text Solution
|
- A nucleus .nX^M emits one alpha particle and one beta-particle. What a...
Text Solution
|
- Select the pairs of isobars and isotones form the following nuclei .6C...
Text Solution
|
- Calculate the energy equivalent of 1 a.m.u. in MeV
Text Solution
|
- One electron volt is the...........when accelerated through a............
Text Solution
|
- Isotopes of an element are the atoms ........... which have..............
Text Solution
|
- Isobars are atoms of..........Which have same............... But diffe...
Text Solution
|
- Isotones are the nuclides which contain............ .
Text Solution
|
- Nuclear forces are the ...............which hold together................
Text Solution
|
- Find the effective mass of a photon if the wavelength of radiation is ...
Text Solution
|
- Natural Chlorine is found to be a mixutre of two isotopes of masses 34...
Text Solution
|