A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-ATOMS AND NUCLEI-Exercise
- M(x) and M(y) denote the atomic masses of the parent and the dougther ...
Text Solution
|
- Heavy stable nuclei have more neutrons than protons. This is because o...
Text Solution
|
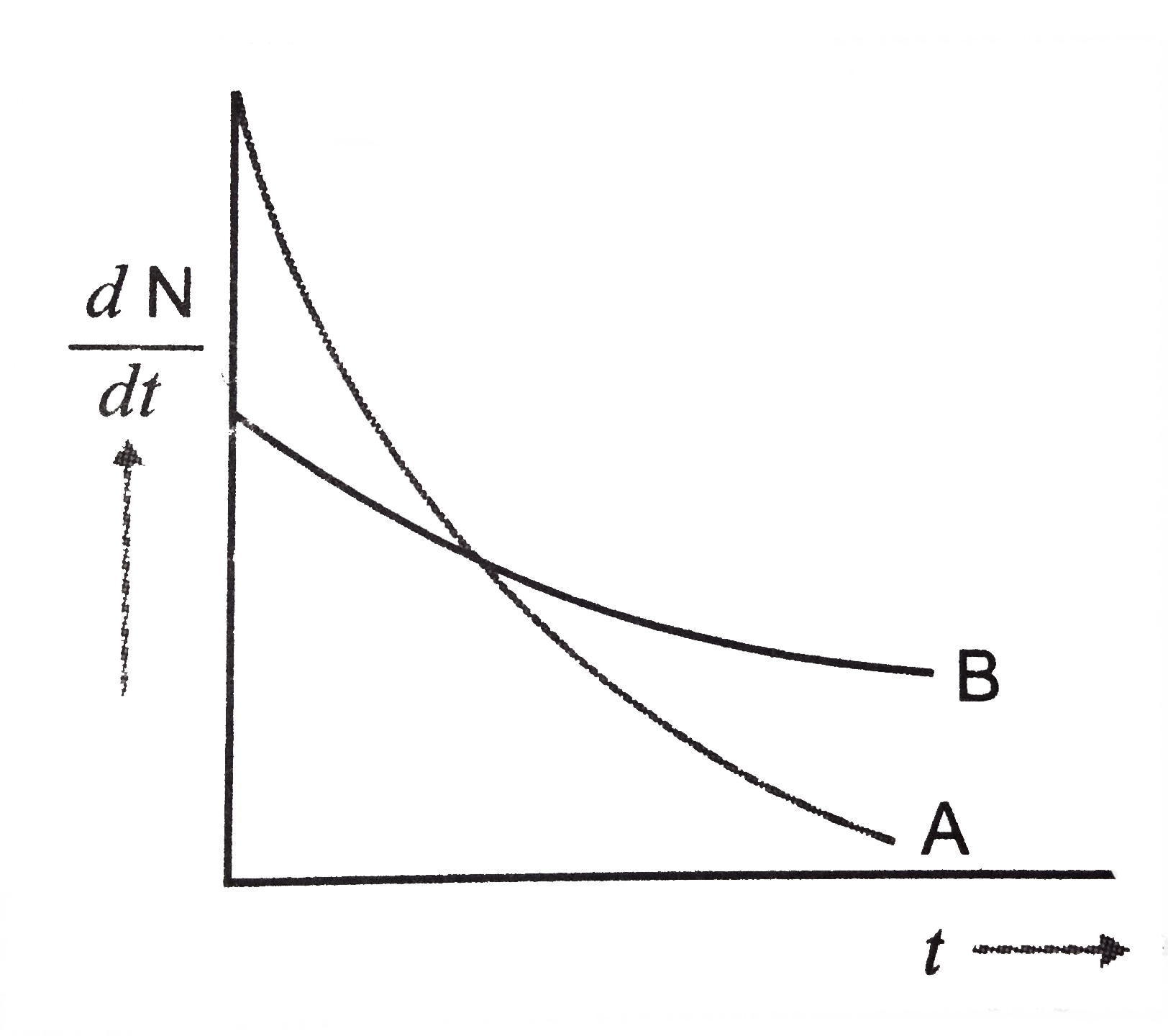
- The variation of decay rate of two radioactive samples A and B with ti...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- In the spectrum of hydrogen atom, the ratio of the longest wavelength ...
Text Solution
|
- Consider a hydrogen atom with its electron in the n^(th) orbital An el...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- The electron in the hydrogen atom jumps form excited state (n=3) to it...
Text Solution
|
- What is the diameter of .(2)Be^(4) in this ground state? Given Bohr ra...
Text Solution
|
- Shows the energy levels for an electron in a certain atom. Which trans...
Text Solution
|
- As the electron in the Bohr orbit is hydrogen atom passes from state n...
Text Solution
|
- A hydrogen atom ia in excited state of principal quantum number n . I...
Text Solution
|
- When an alpha-particle of mass 'm' moving with velocity 'v' bombards o...
Text Solution
|
- Suppose an electron is attracted toward the origin by a force(k)/(r ) ...
Text Solution
|
- The total energy of eletcron in the ground state of hydrogen atom is -...
Text Solution
|
- The energy of a hydrogen atom in the ground state is -13.6 eV. The ene...
Text Solution
|
- The wavelength of the first spectral line in the Balmer series of hydr...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- Some energy levels of a molecule are shown in the fig. The ratio of t...
Text Solution
|
- Out of the following which one is not a possible energy for a photon t...
Text Solution
|