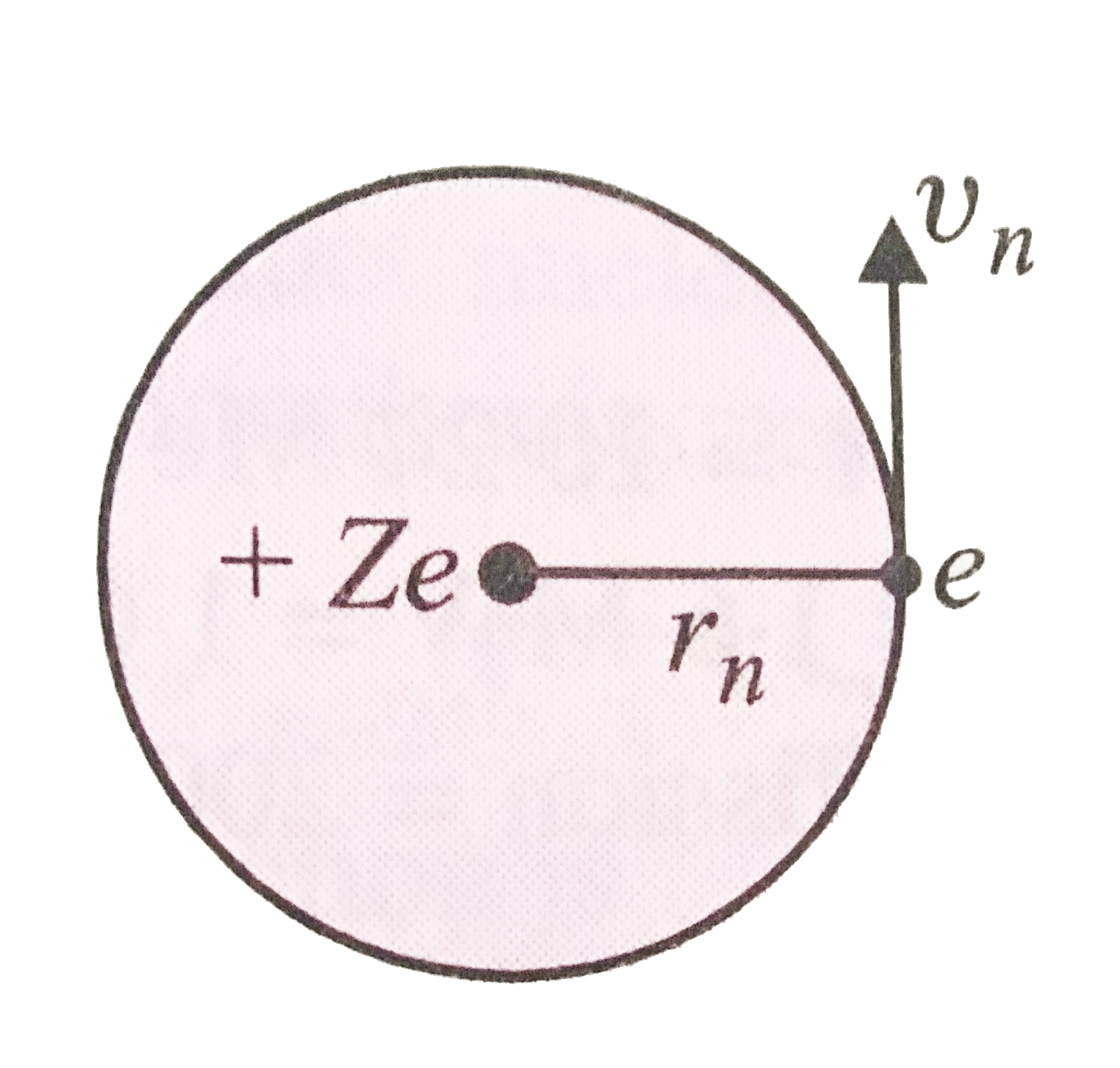A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-ATOMS AND NUCLEI-Exercise
- Hydrogen ((1)H^(1)) Deuterium ((1)H^(2)) singly omised helium ((1)He...
Text Solution
|
- Balmer gives an equation for wavelength of visible radition of H^(-) s...
Text Solution
|
- The kinetic energy of the electron in an orbit of radius r in hydrogen...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- The ratio of wavelength of the lest line of Balmer series and the last...
Text Solution
|
- The radiation corresponding to 3 rarr 2 transition of hydrogen atom f...
Text Solution
|
- if lambda(Cu) is the wavelength of Kalpha, X-ray line fo copper (atomi...
Text Solution
|
- An electron is an excited state of Li^(2 + )ion has angular momentum 3...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- A fission reaction is given by (92)^(236) U rarr(54)^(140) Xe + (38)^(...
Text Solution
|
- The de - Broglie wavelength of a neutron in thermal equilibrium with h...
Text Solution
|
- In the nuclear fusion reaction (1)^(2)H + (1)^(3)H rarr (2)^(4)He + ...
Text Solution
|
- If M(A,Z), M(p)and M(n) denote the masses of the nucleus .(Z)X^(A), pr...
Text Solution
|
- If a star can convert all the He nuclei completely into oxygen nuclei....
Text Solution
|
- On bombardment of U^235 by slow neutrons, 200 MeV energy is released. ...
Text Solution
|
- A nucleus .Z^A X has mass represented by M(A, Z). If Mp and Mp denote ...
Text Solution
|
- In the given reaction .z X^A rarr .(z+1)Y^A rarr .(z-1) K^(A - 4) ra...
Text Solution
|
- The above is a plate of binding energy per nucleon E(0) against the nu...
Text Solution
|
- The power obtained in a reactor using U^235 disintegration is 100 kW. ...
Text Solution
|
- A nucleus .n^ m X emits one alpha-particle and two beta-particles. The...
Text Solution
|