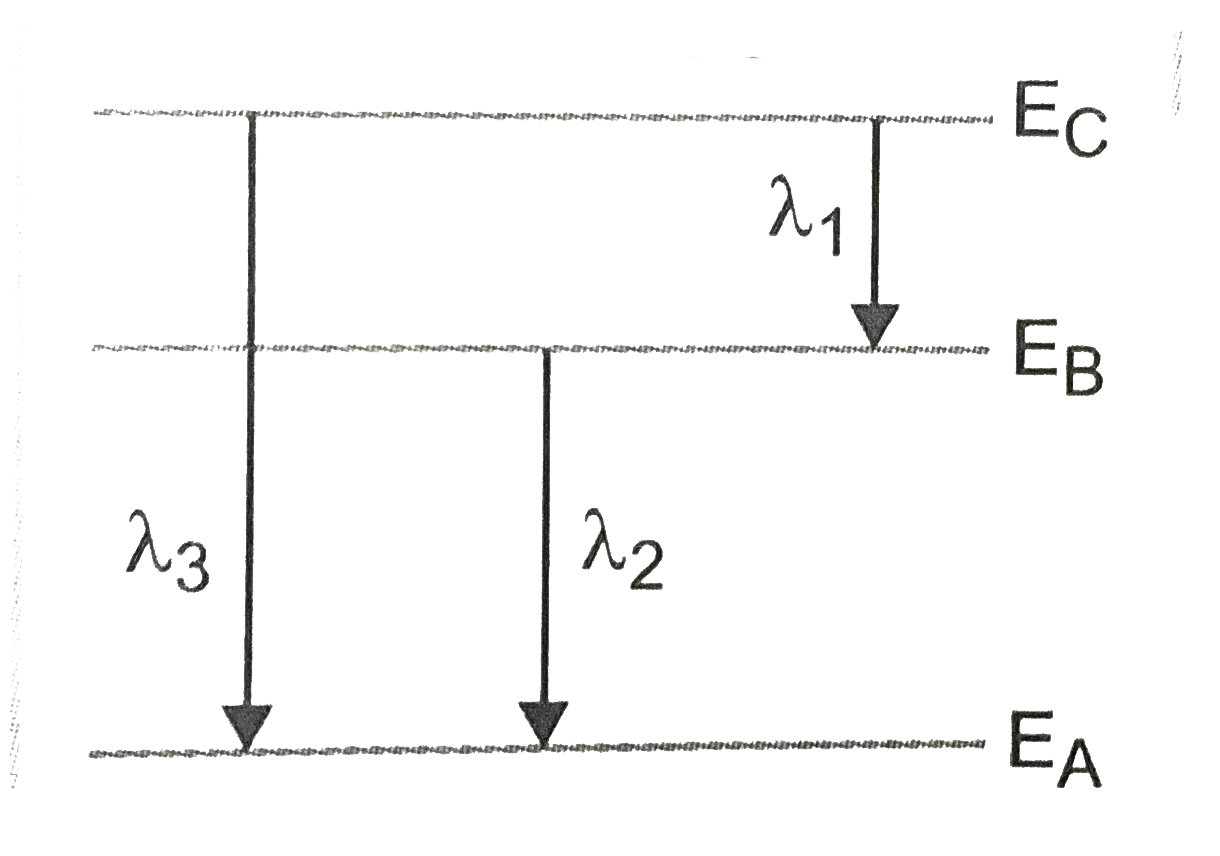
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-ATOMS AND NUCLEI-Exercise
- The half life of Te^(99) is 6h. The activity of Te^(99) in a patient, ...
Text Solution
|
- The radius of first Bohr ortbit is x. The de-Broglie wavelength of ele...
Text Solution
|
- For an atom of an ion having single electron, the wavelengths observed...
Text Solution
|
- Balmer gave an equation for wavelength of visible radiation of H-spect...
Text Solution
|
- A hydrogen atom in its ground state is irradiated by light of waveleng...
Text Solution
|
- Assertion: Balmer series lies in visible region of electromagnetic spe...
Text Solution
|
- Assertion: Lower value of binding energy per nucleon indicates greater...
Text Solution
|
- Assertion : alpha-particle is a helium nucleus. Reason : In alpha-d...
Text Solution
|
- Assertion: Isotopes of an element can be separated by using a mass spe...
Text Solution
|
- Assertion : Rydberg's constant varies with mass no. of a given element...
Text Solution
|
- Assertion: In the process of nuclear fission, the fragments emit two o...
Text Solution
|
- Radioactivity of 108 undecayed radioactive nuclei of half life of 50 d...
Text Solution
|
- Assertion : In a radioactive disintegration, an electron is emitted by...
Text Solution
|
- Assertion : Fragments produced in the fission of U^(235) are active. ...
Text Solution
|
- Electron capture occurs more often than positron emission in heavy ele...
Text Solution
|
- Assertion : An electron and positron can annihilate each other creatin...
Text Solution
|
- Assertion: Forces acting between proton-proton (f(pp)), proton -neutro...
Text Solution
|
- Statement -1 : Large angle scattering of alpha particles led to the di...
Text Solution
|
- Statement-1: Impact parameter for scattering of alpha particles by 180...
Text Solution
|
- Statement-1: Distance of closest approach of alpha particle to the n...
Text Solution
|