Similar Questions
Explore conceptually related problems
Recommended Questions
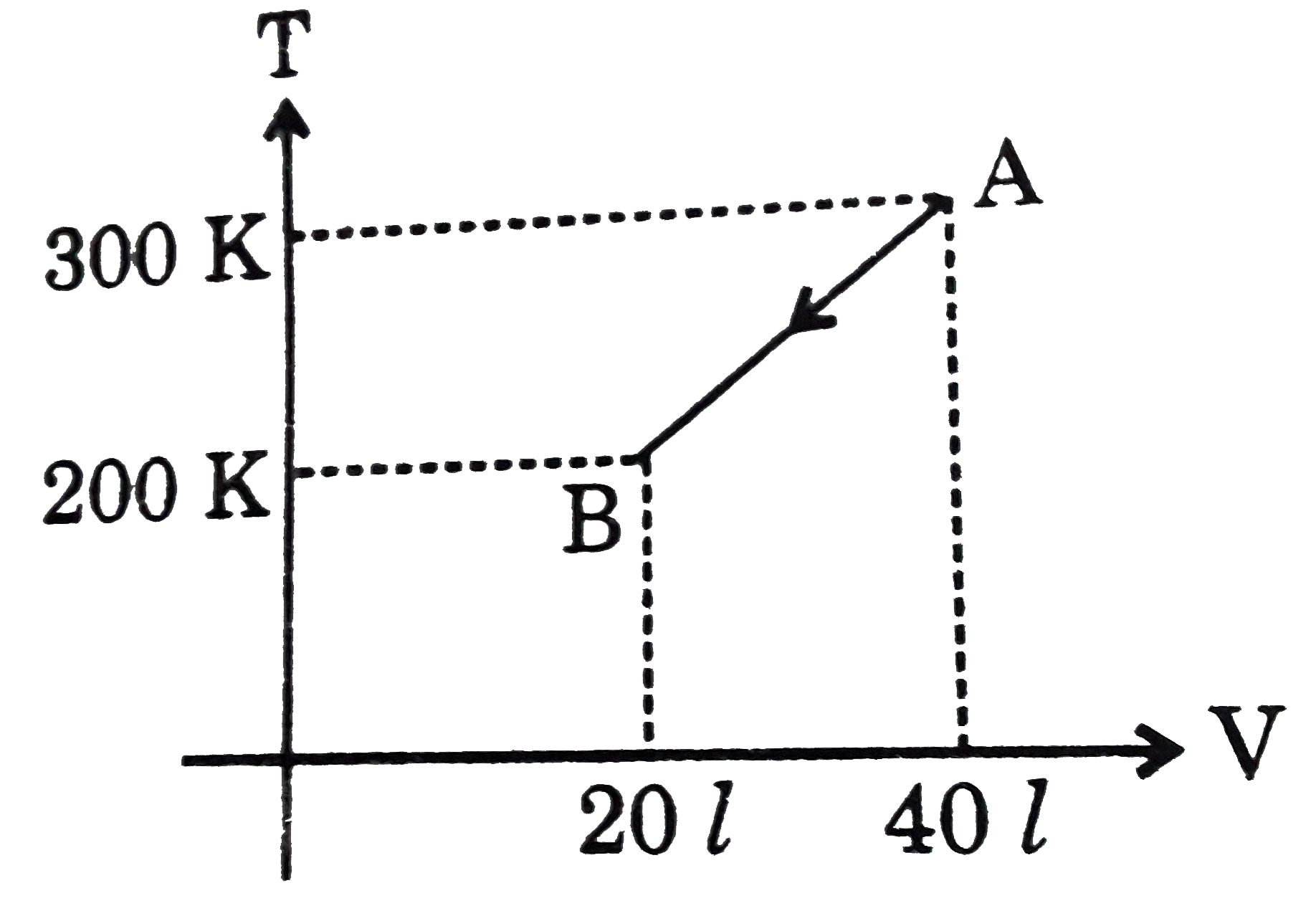
- For 1 mol of an ideal gas work in process AB will be:
Text Solution
|
- The cyclic process for 1 mole of an ideal gas is shown in the V-T diag...
Text Solution
|
- During the process AB of an ideal gas
Text Solution
|
- On a T-P diagram, two moles of ideal gas perform process AB and CD. If...
Text Solution
|
- What will be the work done in process CB for 1 mol of an ideal gas if ...
Text Solution
|
- For 1 mol of an ideal gas work in process AB will be:
Text Solution
|
- Figure demonstrates a polytropic process (i.e.PV^(n) = constant ) for ...
Text Solution
|
- Calculate work done is process BC for 1 mol of an ideal gas if total 6...
Text Solution
|
- 27^(@)C पर एक आदर्श गैस के 1 mol के 2L आयतन को उत्क्रमणीय रूप से प्रसा...
Text Solution
|