A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-CHEMICAL KINETICS-TEST YOUR GRASP
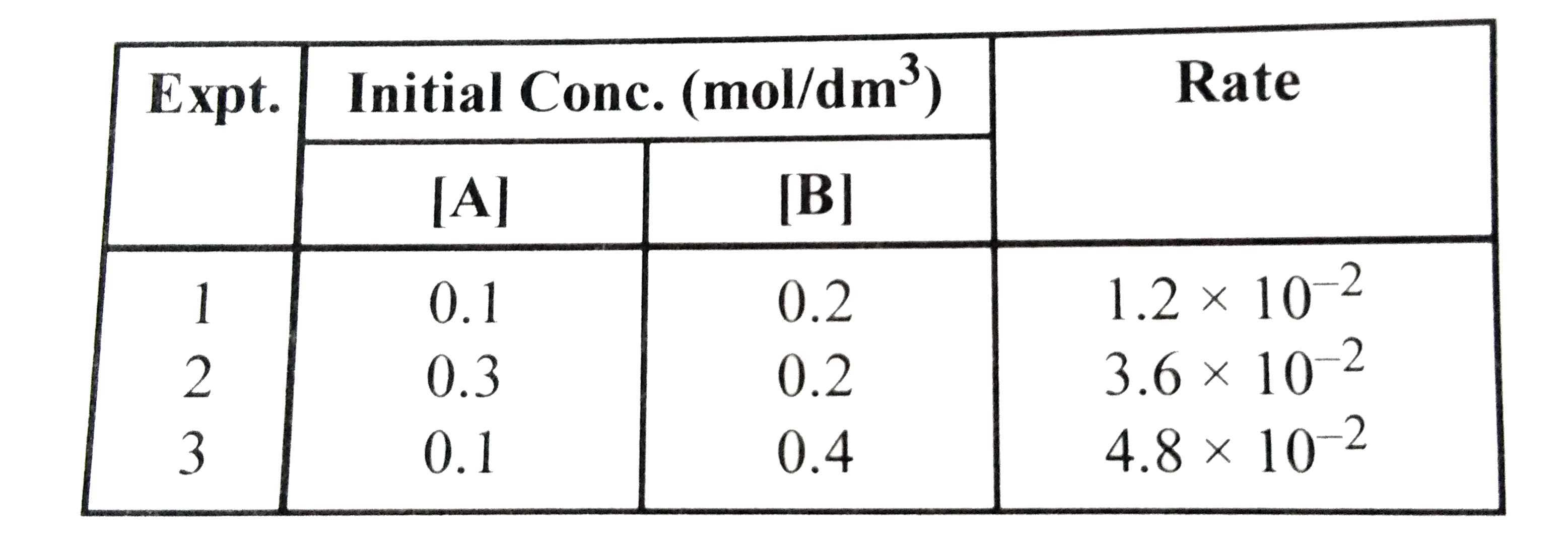
- For the reaction between A and B A+B to product the ...
Text Solution
|
- Chemical kinetics, a branch of physical chemistry, deals with :
Text Solution
|
- The rate of chemical reaction
Text Solution
|
- For the hypothetical reaction 2A rarr3C , the reaction rate r in term...
Text Solution
|
- The term -dx//dt in the rate expresison refers to the
Text Solution
|
- In general, with every 10^(@)C rise in temperature, the rate of reacti...
Text Solution
|
- The activation energy of a reaction is zero. The rate constant of the ...
Text Solution
|
- The activation energy for a hypothetical reaction A rarr X is 12.49 kc...
Text Solution
|
- The rate constant of a reaction depends on
Text Solution
|
- Rate expression of a chemical change is -(dx)/(dt)=k[A]^(2)[B]^(1)[C]^...
Text Solution
|
- If the rate of reaction between A and B is given by rate =k[A][B]^(2) ...
Text Solution
|
- The rate law for the reaction RCl + NaOH(aq) rarr ROH + NaCl is give...
Text Solution
|
- A zero order reactio is one:
Text Solution
|
- For which of the following reactions, the units of rate constant and r...
Text Solution
|
- For a zero order reaction :
Text Solution
|
- If the concentration is expressed in moles per liter, the unit of the ...
Text Solution
|
- Which one of the following formula represents a first-order reaction?
Text Solution
|
- Which of the following is correct plot for effect of catalyst on ...
Text Solution
|
- The specific rate constant of a first order reaction depends on the
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- Which of the following statement regarding the molecularity of a react...
Text Solution
|