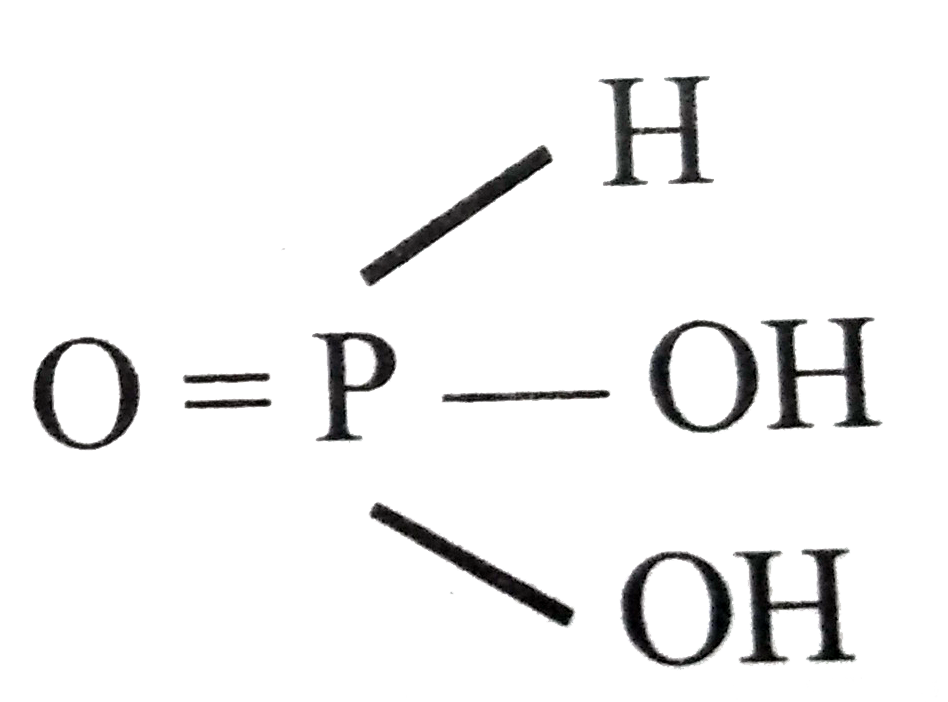A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-P-BLOCK ELEMENTS -TEST YOUR GRASP
- The correct statement for H3PO3 and H3PO4 is
Text Solution
|
- The element which forms oxides in all oxidation states +1 to +5 is.
Text Solution
|
- With Nesslers reagent, ammonia gives brown precipitate due to formatio...
Text Solution
|
- Oxide of nitrogen used as an oxidiser for rocket fuels in missiles and...
Text Solution
|
- Oxide of nitrogen which acts as oxidising as well as reducing agent is
Text Solution
|
- A group 16 element exists in monoatomic state in the metallic lattice....
Text Solution
|
- What is the hybridization of S in SF6 ?
Text Solution
|
- When dipped in conc. HNO3, metals like iron, chromium, nickel, alumini...
Text Solution
|
- Laughing gas has chemical formula
Text Solution
|
- A solution of colourless salt H on boiling with axcess NaOH produces a...
Text Solution
|
- When chloride is passed over dry slaked lime at room temperature the m...
Text Solution
|
- When I(2) is dissolved in C Cl(4), the colour that results is
Text Solution
|
- Fluorine does not show positive oxidation states due to the absence of
Text Solution
|
- The hybridisation of xenon in XeF2 is
Text Solution
|
- An inert gas atoms
Text Solution
|
- Argon is used in arc welding because of its
Text Solution
|
- Nitrogen shows oxidation state +5, but it does not form pentahalide as
Text Solution
|
- PCl3 fumes in moisture because
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- S-S bond is present in
Text Solution
|
- The chemical formula of baryte, the salt of sulphur is
Text Solution
|