A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-D AND F BLOCK ELEMENTS -TEST YOUR GRASP
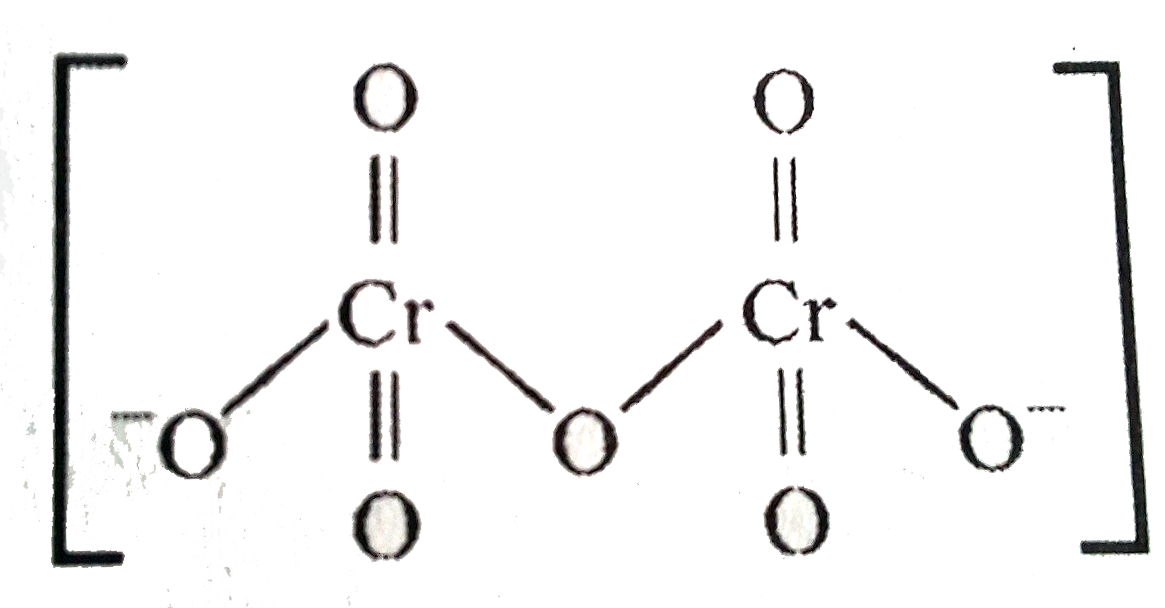
- In the dichromate dianion,
Text Solution
|
- The number of transition series in the periodic table are
Text Solution
|
- What is the general electronic configuration of transition elements
Text Solution
|
- Each transition series contains only 10 elements because
Text Solution
|
- Which one of the following refers to configuration of transition elem...
Text Solution
|
- The outer electronic configuration of chromium is
Text Solution
|
- Which of the following does NOT show different oxidation states?
Text Solution
|
- Calculate the oxidation number of Mn in KMnO(4) molecule.
Text Solution
|
- The properties of transition metals are in between the properties of ...
Text Solution
|
- Which transition metal shows the highest oxidation state ?
Text Solution
|
- Which of the following would be diamagnetic?
Text Solution
|
- The element present immediately below Zn in the periodic table in the ...
Text Solution
|
- Oxidation state of +1 is possible with
Text Solution
|
- For Sc (2 1), if both 3d and 4s electrons in involved in bonding, the ...
Text Solution
|
- Which one of the following forms a colourless solution in aqueous medi...
Text Solution
|
- Which of the following group belongs to transition series ?
Text Solution
|
- In transition elements, the differentiating electron enters into subs...
Text Solution
|
- Which of has the highest number of unpaired electrons?
Text Solution
|
- The range of wavelength of the visible light is
Text Solution
|
- Which of the following salt give coloured aqueous solution ?
Text Solution
|
- Sulphide ore of Zinc is
Text Solution
|