A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES & RADIATION
NIKITA PUBLICATION|Exercise MCQs (Assumption of Kinetic Theory of Gases)|12 VideosKINETIC THEORY OF GASES & RADIATION
NIKITA PUBLICATION|Exercise MCQs (Mean Free Path)|8 VideosINTERFERENCE AND DIFFRACTION
NIKITA PUBLICATION|Exercise MULTPLE CHOICE QUESTIONS|333 VideosMAGNETIC EFFECT OF ELECTRIC CURRENT
NIKITA PUBLICATION|Exercise MCQs (Sensitivity and accuracy of M.C.G.)|1 Videos
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-KINETIC THEORY OF GASES & RADIATION -MCQs (Question Given in MHT-CET)
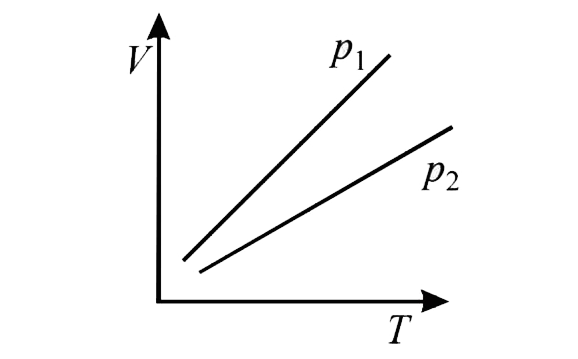
- The volume V versus temperature T graphs for a certain amount of a per...
Text Solution
|
- The difference between the principal specific heats of nitrogen is 300...
Text Solution
|
- The mean kinetic energy of one gram-mole of a perfect gas at abolute t...
Text Solution
|
- The volume of 2.8 g of carbon monoxide at 27^(@) C and 0.821 atm press...
Text Solution
|
- A body cools from 50^(@)C to 46^(@)C in 5 minutes and to 40^(@)C in th...
Text Solution
|
- Two thermometers A and B are exposed in sunlight. The bulb of A is pai...
Text Solution
|
- If at same temperature and pressure, the densities for two diatomic ga...
Text Solution
|
- One mole of an ideal monoatomic gas requires 207 J heat to raise the t...
Text Solution
|
- The volume of a gas at 20^(@)C is 100 cm 3 at normal pressure. If it i...
Text Solution
|
- Radiation emitted by a surface is direclty proportional to
Text Solution
|
- A sphere, a cube and a thin circular plate all made of the same materi...
Text Solution
|
- An ideal gas is that which
Text Solution
|
- 5 gm of air is heated from 273 K to 275 K. the change in internal ener...
Text Solution
|
- Calculate the RMS velocity of molecules of a gas of which the ratio of...
Text Solution
|
- A body at higher temperature T K radiates heat at a rate which is prop...
Text Solution
|
- The wavelength of maximum energy released during an atomic axplosion w...
Text Solution
|
- The root mean square speed of hydrogen molecule at 300 K is 1930m/s. T...
Text Solution
|
- What is the true for 3 moles of a gas?
Text Solution
|
- PV/3=RT, V represents volume of
Text Solution
|
- In a gas 5 molecules have speed 150 m/s, 160 m/s, 170 m/s, 180 m/s, 1...
Text Solution
|
- KE per unit volume is:
Text Solution
|