A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-ELECTRONS AND PHOTONS -MCQs
- It is essential to consider light as a stream of photons to explain
Text Solution
|
- What is the momentum of a photon of frequency v ?
Text Solution
|
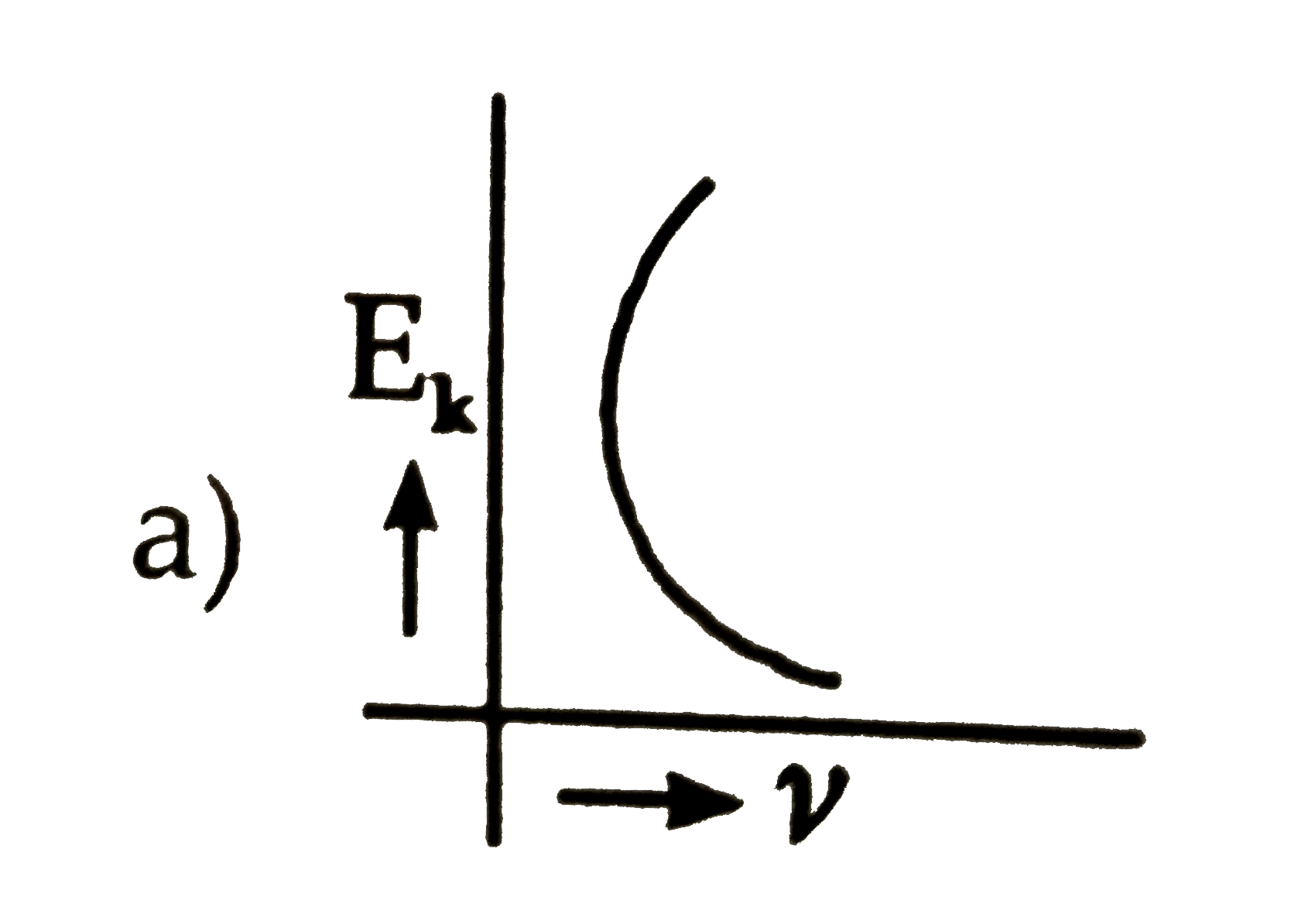
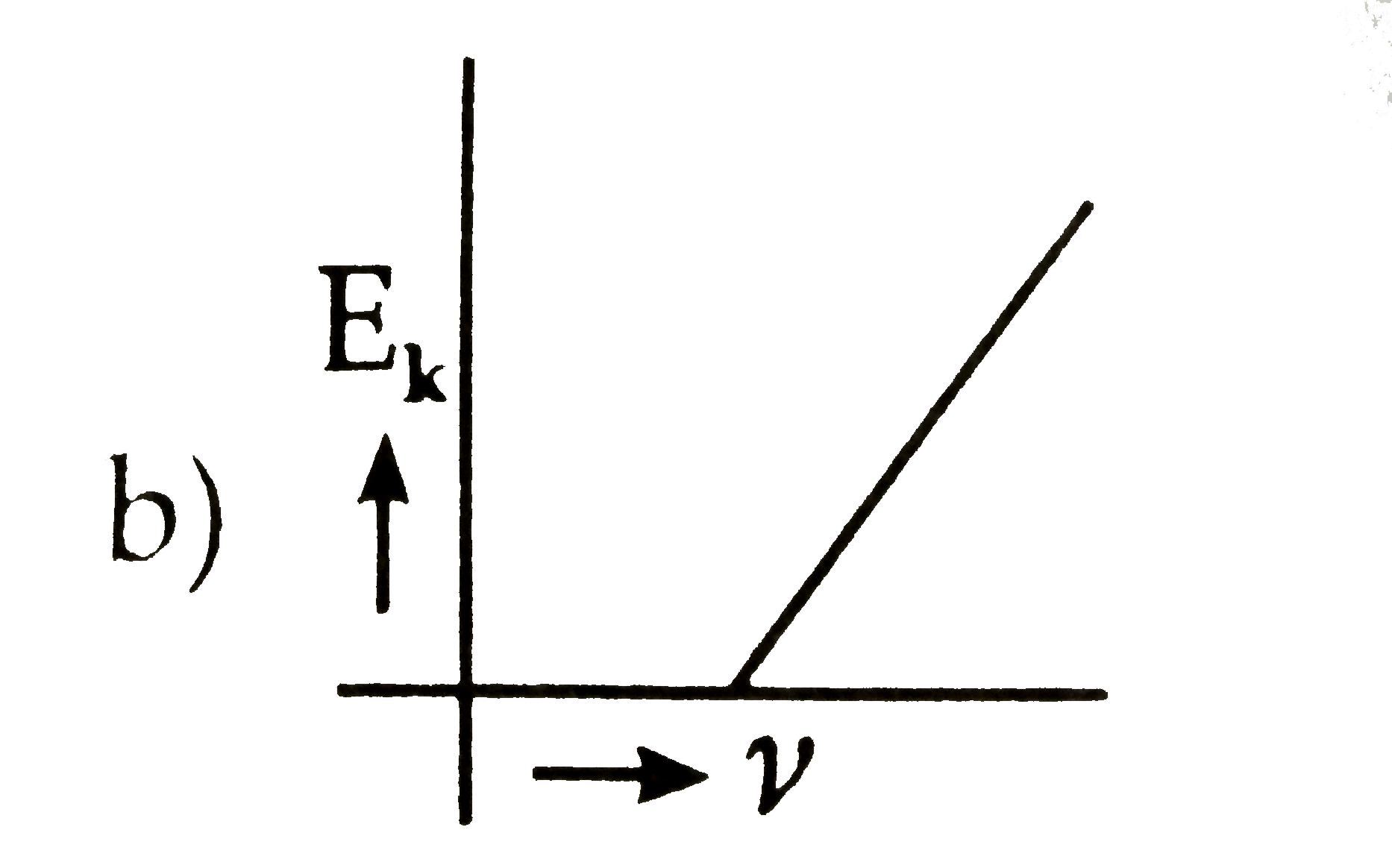
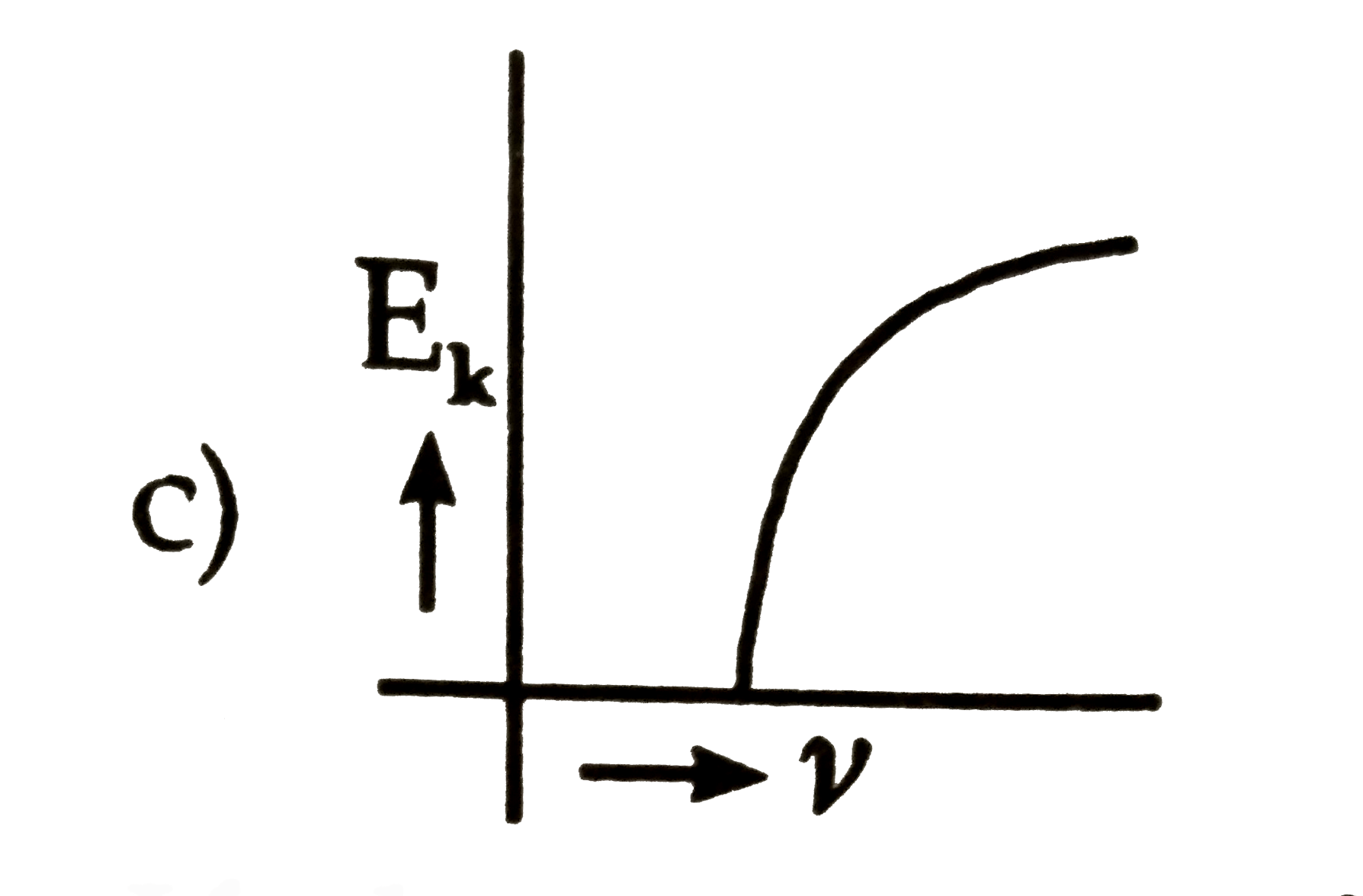
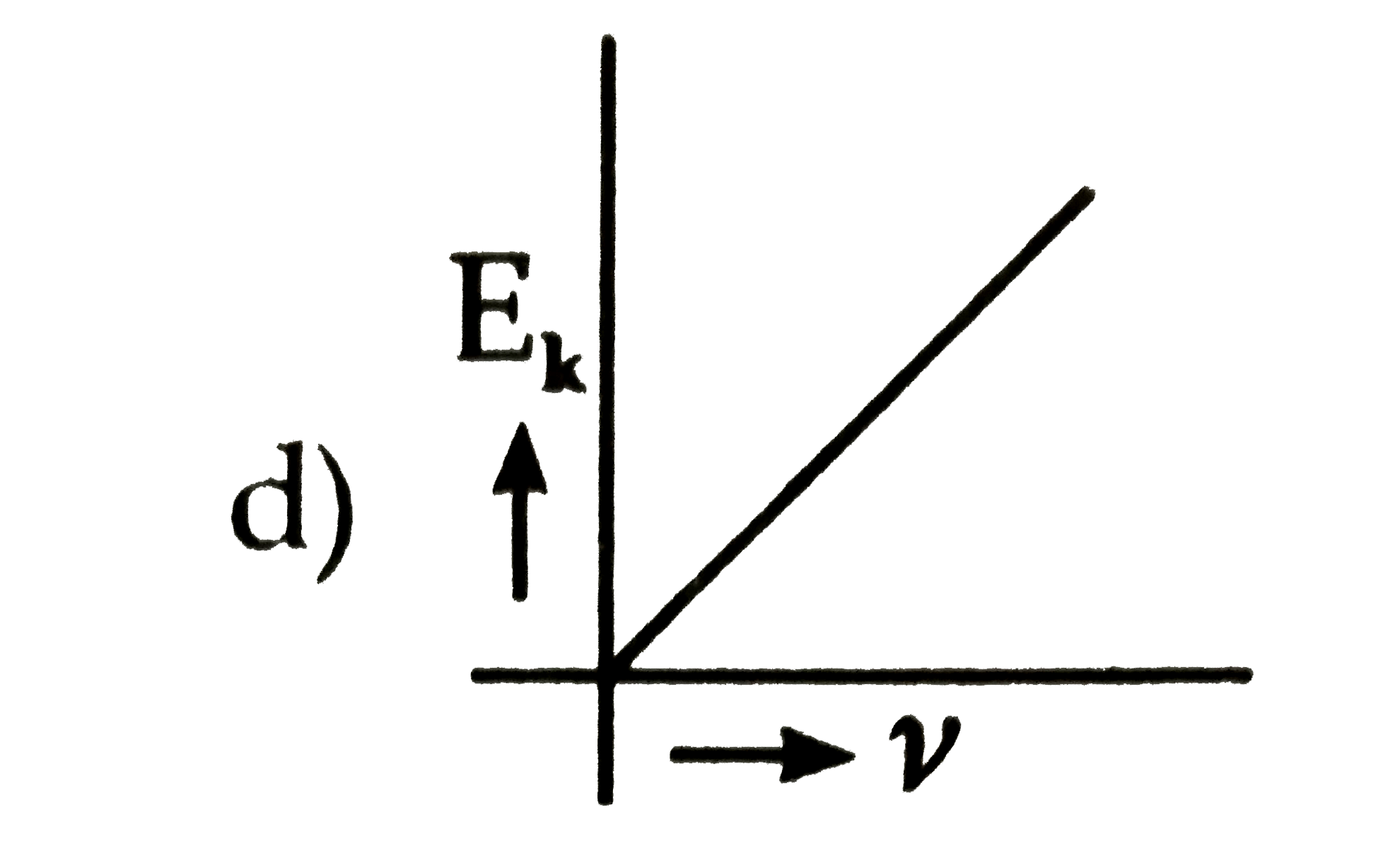
- The kinetic energy (E(k)) of a photoelectron varies with the frequency...
Text Solution
|
- If the energy of a photon corresponding to a wavelength of 6000 Å is 3...
Text Solution
|
- Wavelength of a 1 ke V photon is 1.24 xx 10^(-9) m. What is the freque...
Text Solution
|
- The dual nature of matter was predicted by
Text Solution
|
- The wavelength of an electron , moving with the velocity 3 xx 10^(3) ...
Text Solution
|
- If we consider electrons and photons of same wavelength , then they wi...
Text Solution
|
- The photoelectrons emitted from a given cathode , on the incidence of...
Text Solution
|
- An electron behaves as a
Text Solution
|
- The photons are incident on a metallic surface of frequency n . If the...
Text Solution
|
- An electron moving with a kinetic energy of 1.5 xx 10^(3) eV enters in...
Text Solution
|
- An electron performs uniform circular motion in a region of magnetic f...
Text Solution
|
- An electron of velocity 2.652 xx 10^(7) m/s enters a uniform magnetic ...
Text Solution
|
- In an experiment to measure the charge on an electrons , the electrons...
Text Solution
|
- An electron moving horizontally enters in a uniform electric field of ...
Text Solution
|
- The potential difference through which an electron should be accelerat...
Text Solution
|
- In a velocity selector , the intensity of the electric field is 3 xx 1...
Text Solution
|
- In J.J. Thomson's experiment a potential difference of 600 V is applie...
Text Solution
|
- The threshold wavelength for tungsten is 2730 Å . The work function of...
Text Solution
|