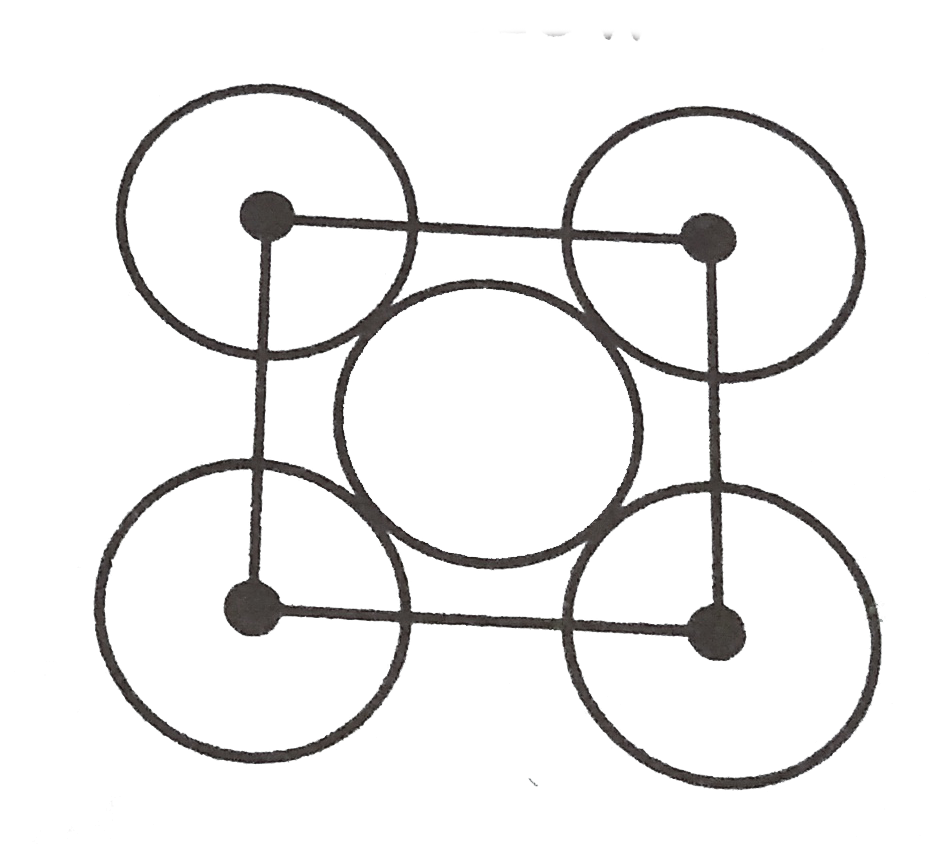
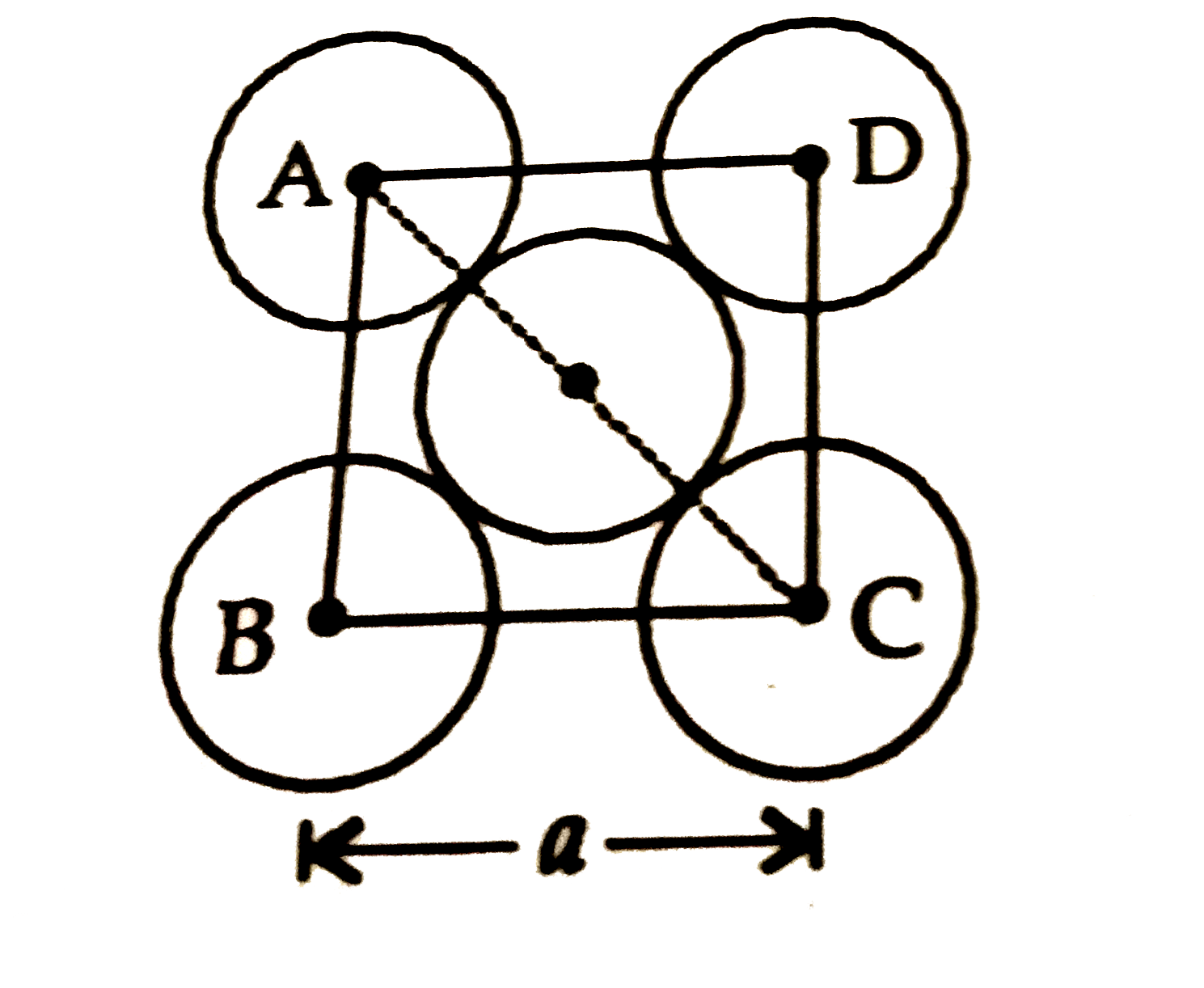A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-SOLID STATE -Question from Competition Exam
- In AgBr crystal , the ion size lies in the order Ag^(+) lt lt Br^(-) T...
Text Solution
|
- The edge length of a face-centred cubic unit cell is 508 pm. If the ra...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- How many nearest neighbours surrounded each particle in a face-centred...
Text Solution
|
- Which of the following dimensio of a unit cell represent a cubic unit
Text Solution
|
- In a face centred cubic lattice, atom A occupies the corner positions ...
Text Solution
|
- In CsCl stricture, the coordination number of Cs^(+) is
Text Solution
|
- In A^(+)B^(-) ionic compound radii of A^(=) and B^(-) ions are 180 "pm...
Text Solution
|
- A solid compound XY has NaCl structure. If the radius of the cation is...
Text Solution
|
- Copper crystallises in fcc with a unit cell length of 361 pm. What is ...
Text Solution
|
- If the unit length of the unit cell is 5 Å the smallest distance is ...
Text Solution
|
- The total number of octahedral void (s) per atom present in a cubic cl...
Text Solution
|
- A metal crystallizes with a face-centered cubic lattice. The edge of t...
Text Solution
|
- Lithium forms body centred cubic structrue. The length of the side of...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- Barium titanate has the pervoskite structure, i.e. a cubinc lattice wi...
Text Solution
|
- In a face centred cubic lattice, a unit cell is shared equally by low ...
Text Solution
|
- The distance is picometer between centre of two closest sodium atoms i...
Text Solution
|
- With respect to graphite and diamond, which of the statement given is...
Text Solution
|
- Which of the following exists as covalent crystals in the solid state?
Text Solution
|