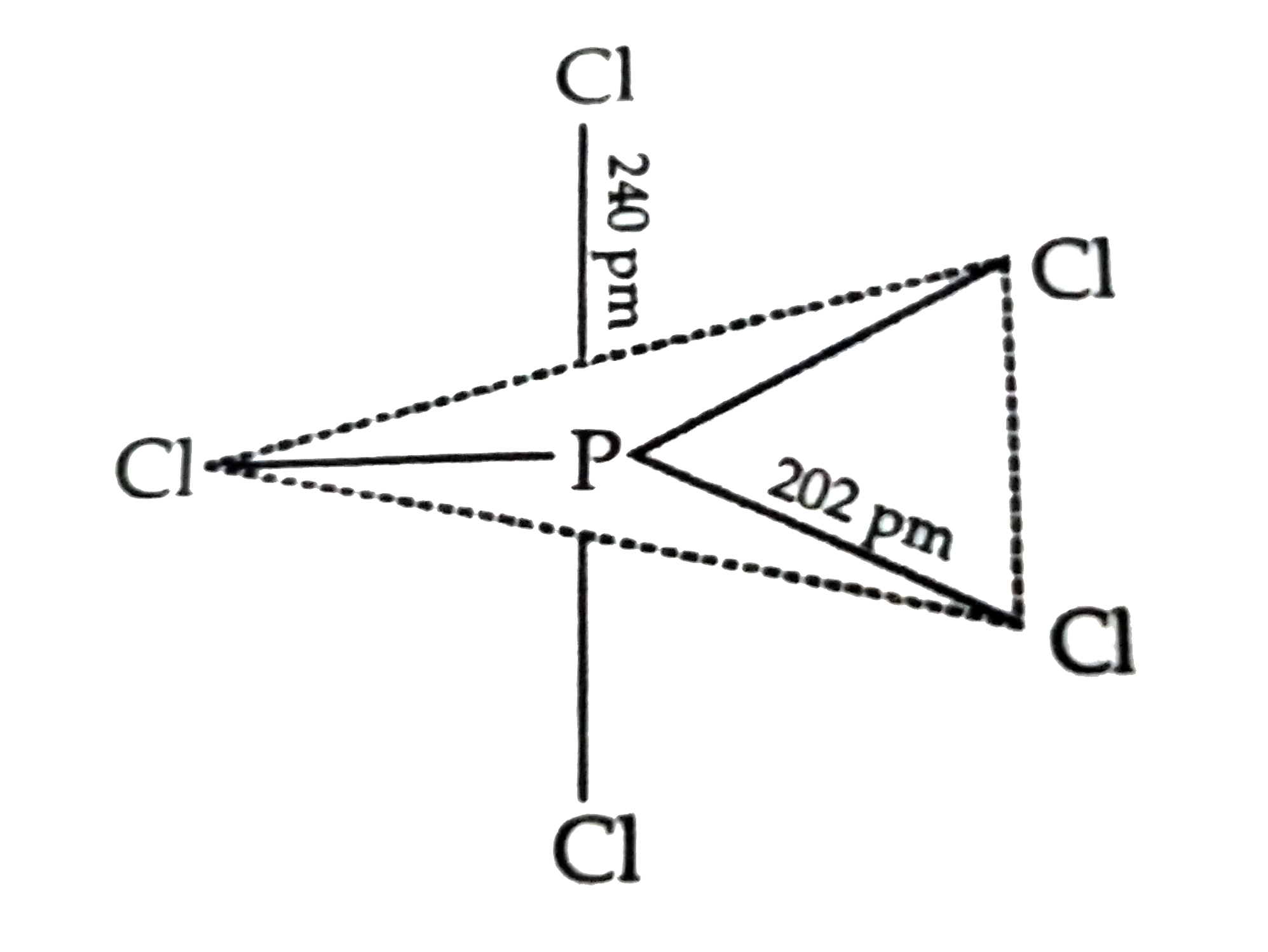
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-P-BLOCK ELEMENT -Competitive Exam
- Pick out the incorrect statement
Text Solution
|
- In which of the following arrangements, the sequence is not strictly a...
Text Solution
|
- Each of the following is true for white and red phosphorus except that...
Text Solution
|
- Which of the following is not known
Text Solution
|
- Which one of the following elements is most metallic?
Text Solution
|
- Which of the following oxides of nitrogen is the anhydride of HNO(3)?
Text Solution
|
- Dissociatuion of H(3)PO(4) occurs in following stages
Text Solution
|
- Nitrogen forms how many oxides
Text Solution
|
- The element which catches fire in air at 30^@C and is stored under wat...
Text Solution
|
- A solution of ammonia in water contains
Text Solution
|
- Which of the following is oxidised in air?
Text Solution
|
- Which of the following exist in polymeric form ?
Text Solution
|
- Which nitrogen trihalides is least basic
Text Solution
|
- Which of the following oxide of nitrogen is a coloured gas?
Text Solution
|
- Which oxide does not act as a reducing agent?
Text Solution
|
- The basic character of hydrides of the 15th-group elements decreases i...
Text Solution
|
- The strongest base is
Text Solution
|
- Which has the lowest boiling point?
Text Solution
|
- Of the following, the most acidic is
Text Solution
|
- The correct order of the acidic nature of oxides is in the order
Text Solution
|
- Nitrogen is relatively inactive element because is atom has a stable e...
Text Solution
|