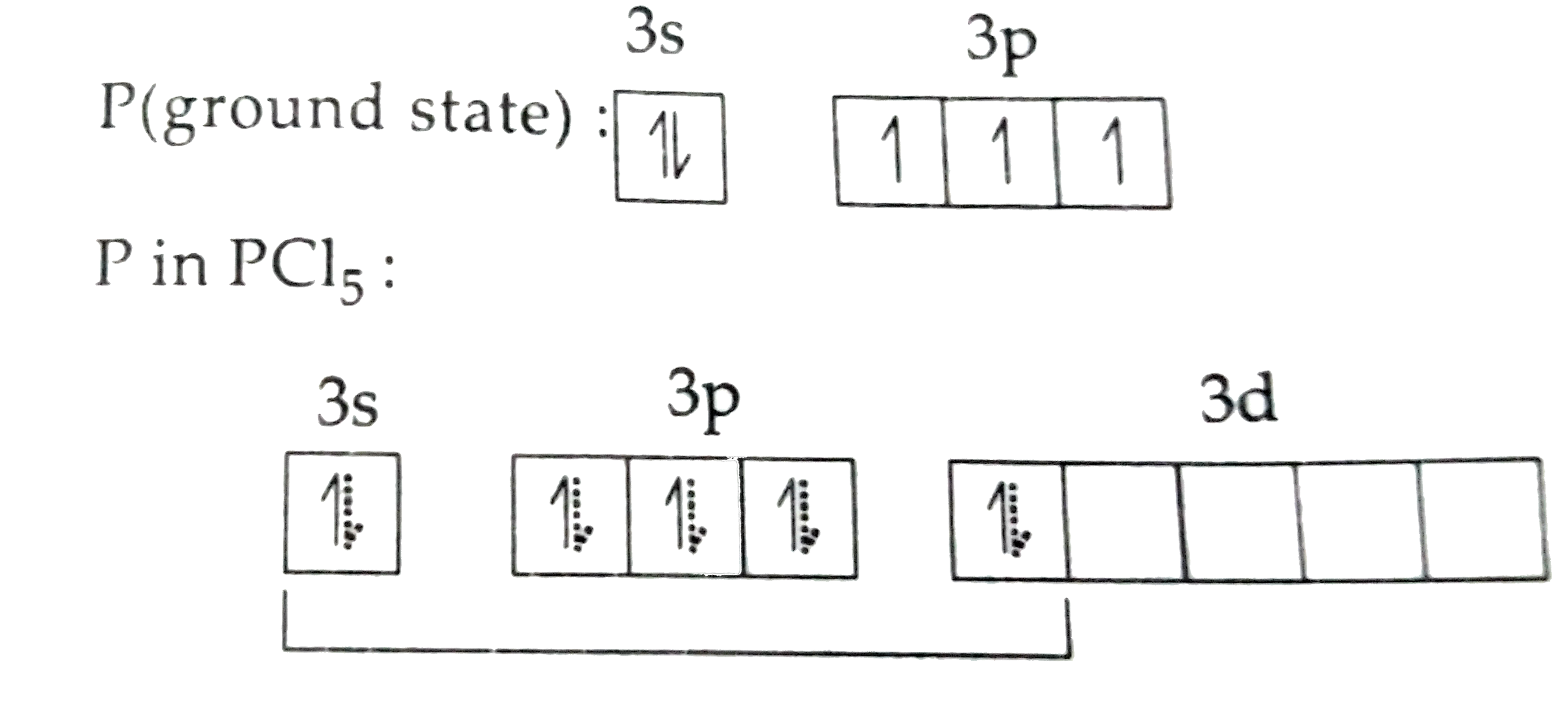
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-P-BLOCK ELEMENT -Competitive Exam
- The structural formula of hypophosphorous acid is
Text Solution
|
- Aquaregia is
Text Solution
|
- Which of the following set of properties belong to PCI(5)?
Text Solution
|
- In NH(3) and PH3, the common is
Text Solution
|
- CaC(2)+N(2) to A, "Product A is "
Text Solution
|
- In compounds of type ECI(3), where E=BP, As or B, the angles CI-E-CI f...
Text Solution
|
- An element (X) forms compounds of the formuls XCl(3),X(2)O(5) and Ca(3...
Text Solution
|
- Which of the following statement is not valid for oxoaids of phosphoru...
Text Solution
|
- Which of the following oxyacids of phosphorus is a reducing agent and ...
Text Solution
|
- The element which forms oxides in all oxidation states +1 to +5 is.
Text Solution
|
- The tota number of P-O bonds in P(4)O(10) is
Text Solution
|
- Most acidic oxide is
Text Solution
|
- White phosphorus is
Text Solution
|
- Sulphur molecule is converted into sulphur ion, when it
Text Solution
|
- Industrial name for H2S2O7 is
Text Solution
|
- The number of unpaired electrons in the p-subshell of oxygen atom
Text Solution
|
- Electron affinity is positive when
Text Solution
|
- Which of the following is most electronegative?
Text Solution
|
- Which of the following statements regarding sulphur is incorrect?
Text Solution
|
- Bond angle is minimum for
Text Solution
|