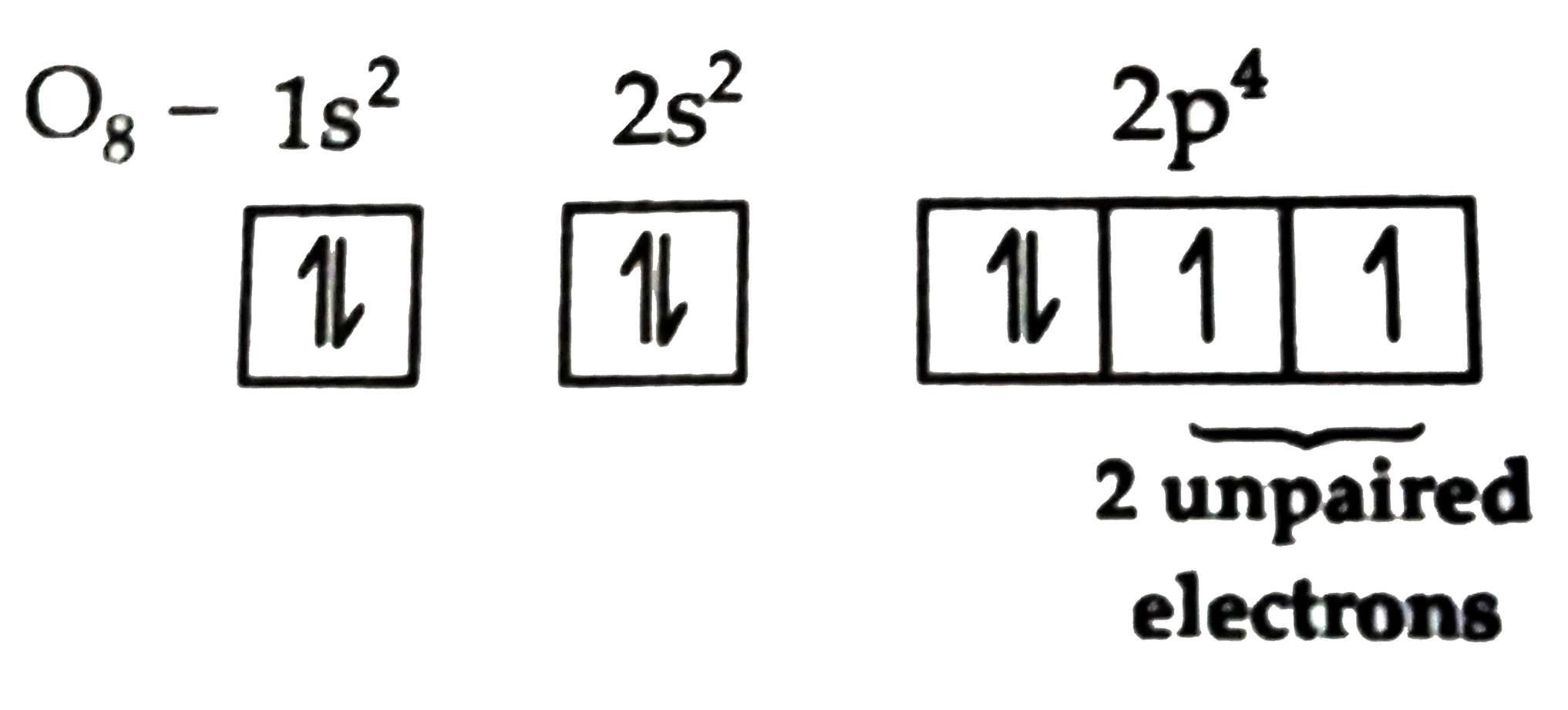A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-P-BLOCK ELEMENT -Competitive Exam
- Sulphur molecule is converted into sulphur ion, when it
Text Solution
|
- Industrial name for H2S2O7 is
Text Solution
|
- The number of unpaired electrons in the p-subshell of oxygen atom
Text Solution
|
- Electron affinity is positive when
Text Solution
|
- Which of the following is most electronegative?
Text Solution
|
- Which of the following statements regarding sulphur is incorrect?
Text Solution
|
- Bond angle is minimum for
Text Solution
|
- Oxygen molecule exhibits
Text Solution
|
- Ozone is obtained from oxygen
Text Solution
|
- Ozone with K solution produces
Text Solution
|
- When H2S is passed through acidified KMNO4, we get
Text Solution
|
- Copper turnings when heated with concebtracted sulphuric acid will giv...
Text Solution
|
- Which compounds acts as an oxidising as well as reducing agent?
Text Solution
|
- A solution of sulphur dioxide in water reacts with H(2)S precipitating...
Text Solution
|
- When SO(2) is passed through acidified K(2)Cr(2)O(7) solution
Text Solution
|
- Bleaching action of SO(2) is due to and is
Text Solution
|
- A salt of sulphures acid is called
Text Solution
|
- Which of the following is acidic?
Text Solution
|
- The final acid obtained during the manufacturing of H(2)SO(4) by conta...
Text Solution
|
- In the reaction 2Ag+2H(2)SO(4)rarrAg(2)SO(4)+2H(2)O+SO(2),H(2)SO(40act...
Text Solution
|