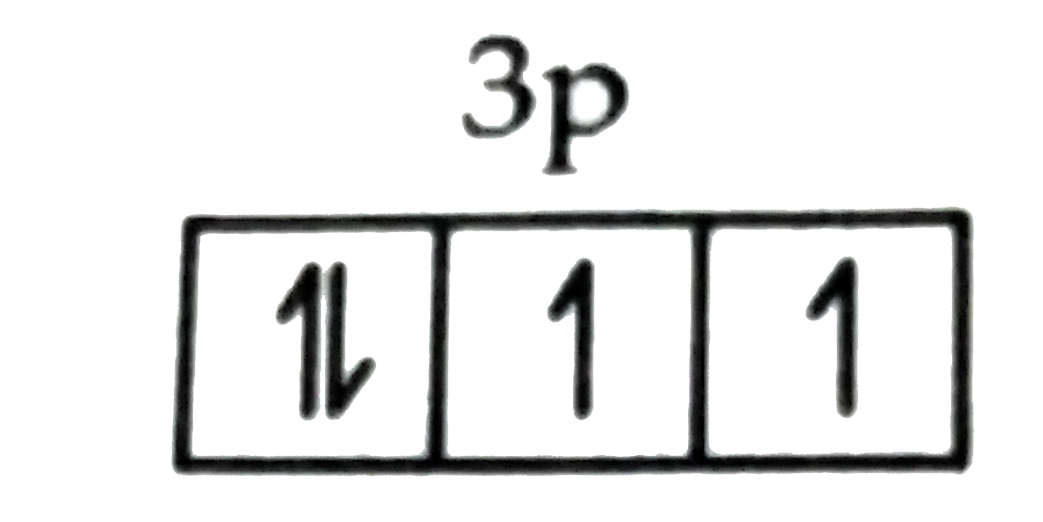A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-P-BLOCK ELEMENT -Competitive Exam
- Which one of the gas dissolves in H(2)SO(4) to give oleum?
Text Solution
|
- There is no S-S bond in
Text Solution
|
- Number of unpaired electrons in sulphur is
Text Solution
|
- Which of the following mixture gives chromic
Text Solution
|
- The gas used in artificial respiration is.
Text Solution
|
- Amongest H(2)O,H(2)S,H(2)Se and H(2)Te the one with highest boiling p...
Text Solution
|
- Which of the following dissociates to give H^(+) most easily?
Text Solution
|
- Among KO2 , AlO(2)^(-) BaO2 and NO2^+ unpaired electron is present in...
Text Solution
|
- Point out in which of the following properties oxygen differs from the...
Text Solution
|
- Whichg of the following hydrides has the lowest boiling point?
Text Solution
|
- The catalyst used in the manufacture of H(2)SO(4) by contact process i...
Text Solution
|
- Which of the following acts as pickling agent ?
Text Solution
|
- When H2S gas in passed through nitric acid, the product is :
Text Solution
|
- Shape of O2F2 is similar to that of
Text Solution
|
- Which of the following is not a chalcogen?
Text Solution
|
- Permono sulphuric acid is known as
Text Solution
|
- KO(2)+CO(2)rarr?("gas")
Text Solution
|
- Peroxydisulphuric acid has the following bond
Text Solution
|
- In presence of moisture, SO(2) can
Text Solution
|
- A gas that cannot be collected over water is.
Text Solution
|