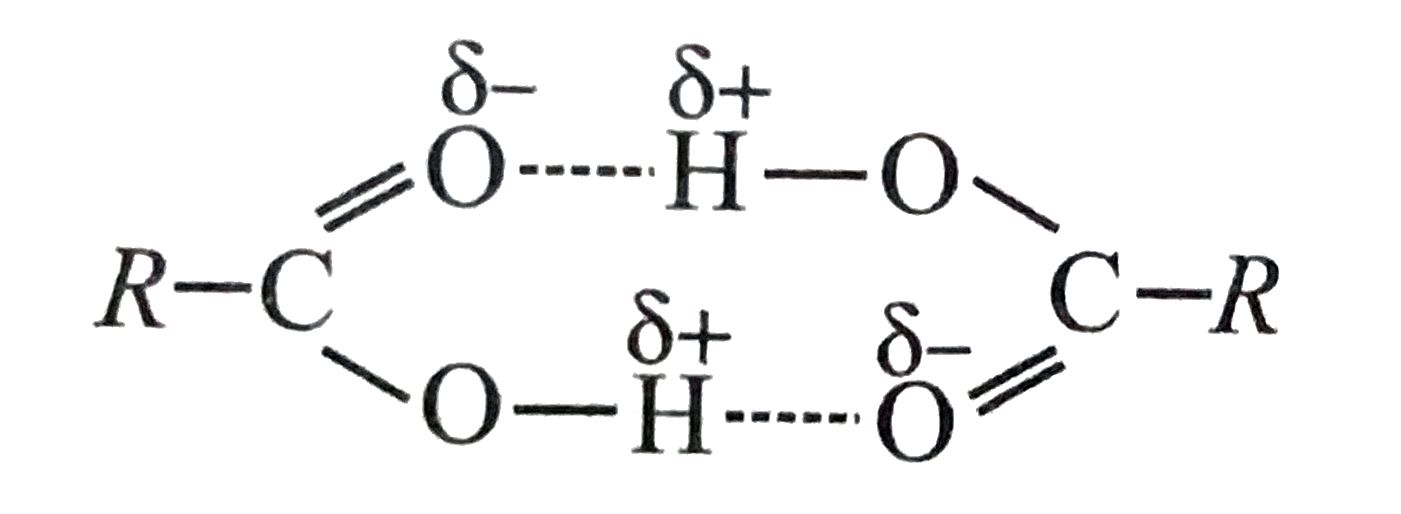A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-Assertion And Reason
- Assertion : In formaldehyde all the four atoms lie in one plane. Reas...
Text Solution
|
- Assertion : Acetaldehyde can be prepared by addition of water to ethy...
Text Solution
|
- Assertion : Etard reaction helps to stop the oxidation of toluene at t...
Text Solution
|
- Assertion : The boiling points of given compounds follow the order : ...
Text Solution
|
- Assertion : Acetaldehyde is more reactive than acetone in nucleophilic...
Text Solution
|
- Assertion : beta- hydrogen atom of carbonyl compounds is acidic in nat...
Text Solution
|
- Assertion : Cross aldol condensation of ethanal and propanal gives a m...
Text Solution
|
- Assertion : Aromatic aldehydes and formaldehyde undergo Cannizzaro rea...
Text Solution
|
- Assertion : Aromatic aldehydes and ketones undergo electrophilic subst...
Text Solution
|
- Assertion : In the presence of alkaline KMnO(4) 4-methylacetophenone ...
Text Solution
|
- Assertion : Most carboxylic acids exist as dimers in the vapour phase ...
Text Solution
|
- Assertion : Phenol and benzoic acid can be distinguished by Na(2)CO(3)...
Text Solution
|
- Assertion : Direct attachment of groups such as phenyl or vinyl to the...
Text Solution
|
- Assertion : (CH(3))(3)C COOH does not give HVZ reaction. Reason : (C...
Text Solution
|
- Assertion : Carboxylic acids do not undergo Friedel - Crafts reaction....
Text Solution
|