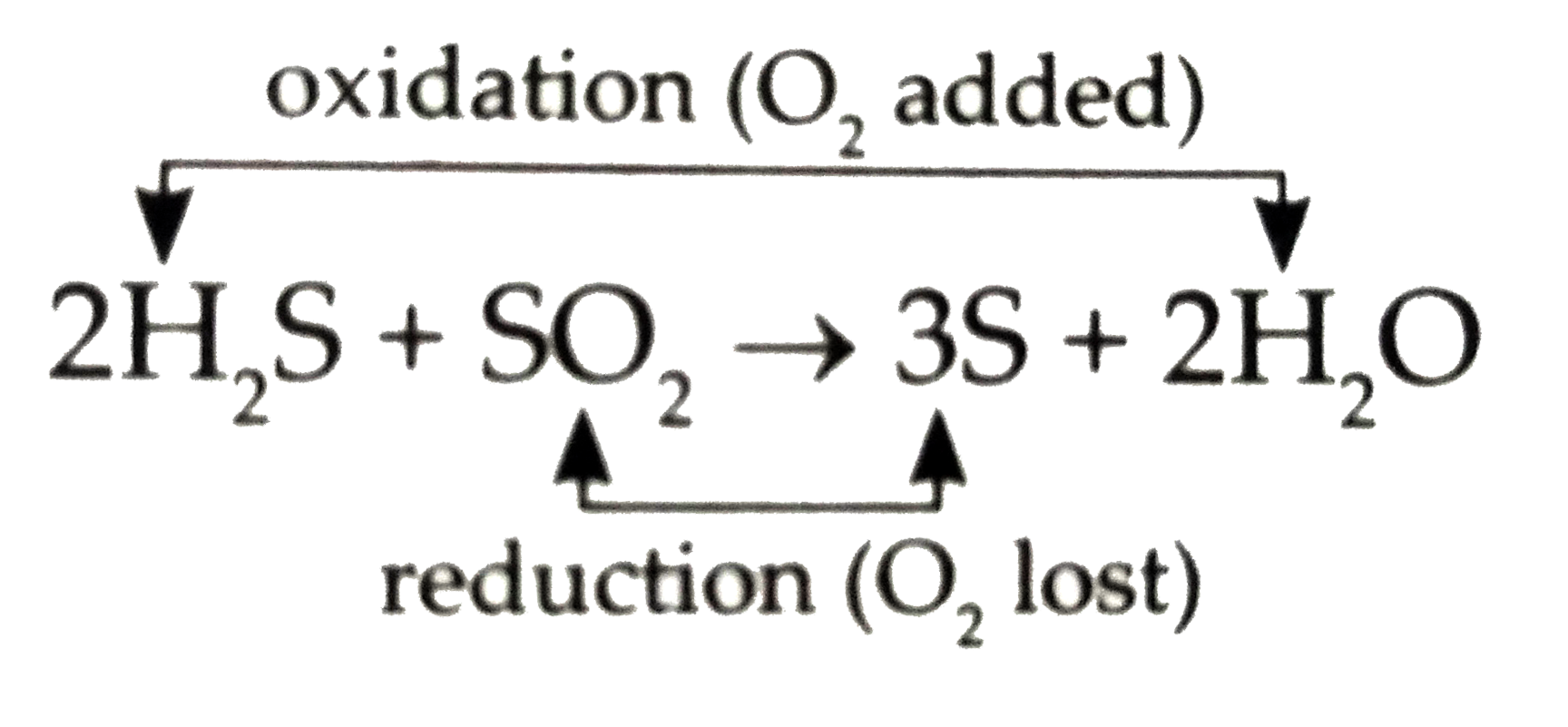Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise DEFINE THE FOLLOWING/WRITE NOTES:|17 VideosCHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise COMPLETE THE FOLLOWING CHEMICAL EQUATIONS, BALANCE AND STATE THE TYPE OF REACTIONS|6 VideosCHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise CHOOSE AND WRITE THE CORRECT OPTION|13 VideosCARBON COMPOUNDS
CHETAN PUBLICATION|Exercise Answer the following (Any 1)|2 VideosMETALLURGY
CHETAN PUBLICATION|Exercise ASSIGNEMENT -21|1 Videos
Similar Questions
Explore conceptually related problems
CHETAN PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-ANSWER THE FOLLOWING
- What is the number of molecules of reactants taking part in the above ...
Text Solution
|
- How many products are formed in each of the above reaction ?
Text Solution
|
- Write the electronic concept of oxidation and reduction reactions.
Text Solution
|
- The rate of decomposition of hydrogen peroxide decreases in presence o...
Text Solution
|
- Define reactant and product.
Text Solution
|
- Oxygen, hydrogen, and sulphur are examples for?
Text Solution
|
- Explain the similarity and difference in two events, namely adding NaO...
Text Solution
|
- What is rancidity ?Mention only two ways which rancidity can be preve...
Text Solution
|
- Why is it easier for you to float in salt water than in fresh water?
Text Solution
|
- Identify from the following reaction the reactsnat that undergo oxidat...
Text Solution
|
- Classify the reactions into different type. AgNO(3(aq)) + NaCl((s))t...
Text Solution
|
- Classify the reactions into different type. CaO((s) + H(2)O ((l))to ...
Text Solution
|
- Classify the reactions into different type. 2KClO(3(s)) overset(Delt...
Text Solution
|
- Classify the reactions into different type. 2KClO(3(s)) overset(Delt...
Text Solution
|
- Classify the reactions into different type. CuSO(4(aq))+Pb((s))to Pb...
Text Solution
|
- Classify the reactions into different type. 2H(2) O(2) underset("lig...
Text Solution
|
- Classify the reactions into different type. BaS((aq))+ZnSO(4(aq))to ...
Text Solution
|
- Balance the equation stepwise. H(2)S(2)O(7(l))+ H(2)O((l))ot H(2)SO(...
Text Solution
|
- Balance the equation stepwise. Write down the physical states of rea...
Text Solution
|
- Balance the equation stepwise. Ag((s)) + HCl((aq))to AgCl darr +H(2)...
Text Solution
|