Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise DEFINE THE FOLLOWING/WRITE NOTES:|17 VideosCHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise COMPLETE THE FOLLOWING CHEMICAL EQUATIONS, BALANCE AND STATE THE TYPE OF REACTIONS|6 VideosCHEMICAL REACTIONS AND EQUATIONS
CHETAN PUBLICATION|Exercise CHOOSE AND WRITE THE CORRECT OPTION|13 VideosCARBON COMPOUNDS
CHETAN PUBLICATION|Exercise Answer the following (Any 1)|2 VideosMETALLURGY
CHETAN PUBLICATION|Exercise ASSIGNEMENT -21|1 Videos
Similar Questions
Explore conceptually related problems
CHETAN PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-ANSWER THE FOLLOWING
- Balance the equation stepwise. H(2)S(2)O(7(l))+ H(2)O((l))ot H(2)SO(...
Text Solution
|
- Balance the equation stepwise. Write down the physical states of rea...
Text Solution
|
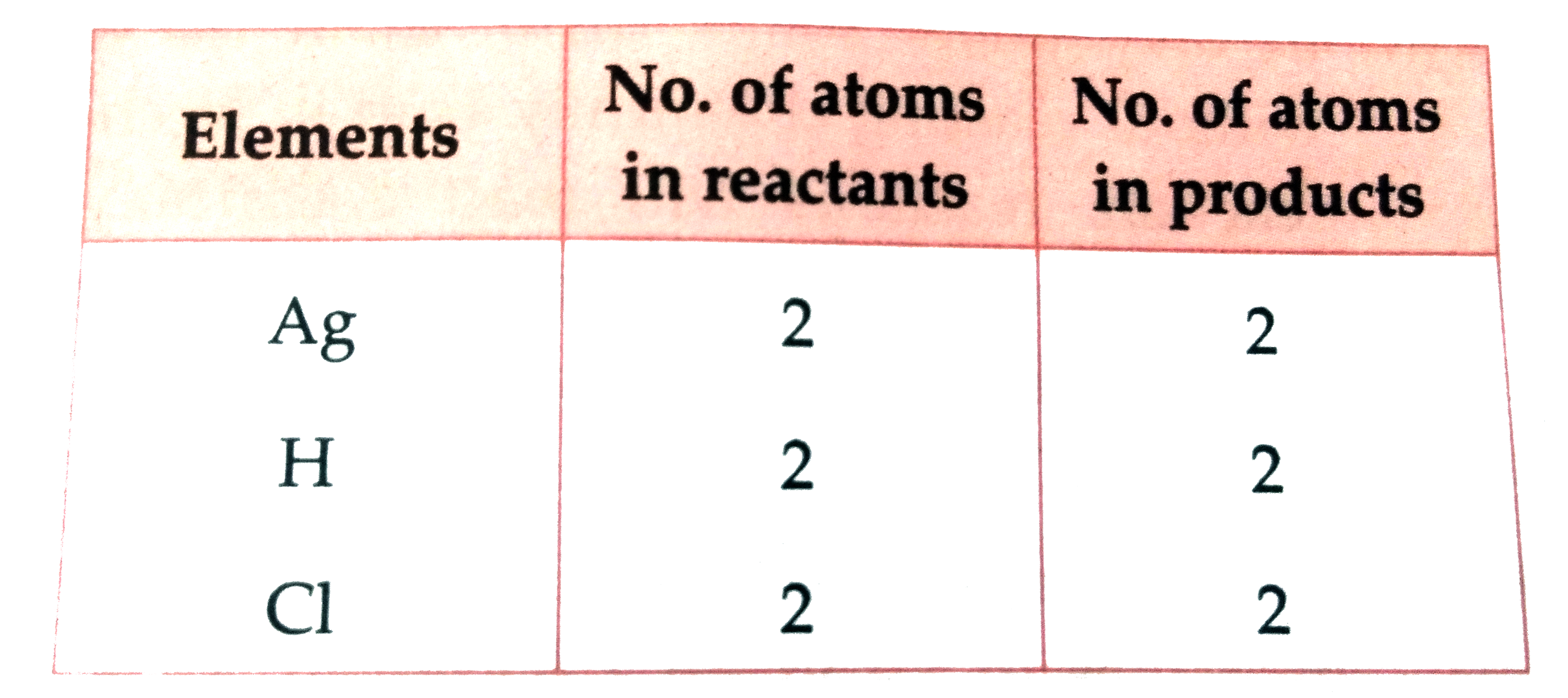
- Balance the equation stepwise. Ag((s)) + HCl((aq))to AgCl darr +H(2)...
Text Solution
|
- Balance the equation stepwise. NaOH((aq)) +H(2)SO(4(aq))to Na(2) SO(...
Text Solution
|
- Balance the equation stepwise. Write down the physical states of rea...
Text Solution
|
- Write balanced chemical equation for the following processes evapora...
Text Solution
|
- Balance the equation stepwise. Write down the physical states of rea...
Text Solution
|
- Balance the equation stepwise. Write down the physical states of rea...
Text Solution
|
- Balance the equation stepwise. Is it posible to produce hydrogen by ...
Text Solution
|
- Define a chemical reaction.
Text Solution
|
- is the substance that is dissolved in a solvent.
Text Solution
|
- Balance the equation stepwise. Take into account the time required f...
Text Solution
|
- Complete the reaction and give names of the products. CuSO(4(aq))+ Fe(...
Text Solution
|
- Complete the reaction and give names of the products. CuSO(4(aq)) +P...
Text Solution
|
- Observe the following picture write down the chemical reaction with ex...
Text Solution
|
- Iron sulphide reacts with sulphureic acid.
Text Solution
|
- what's happened when Zinc dust is added to copper sulphate solution.?
Text Solution
|
- How many moles of barium suphate is precipitated when 1 mole of alumin...
Text Solution
|
- What happens when excess of CO(2) reacts with calcium carbonate?
Text Solution
|
- (i) Write the products formed in the reaction of concentrated nitric a...
Text Solution
|