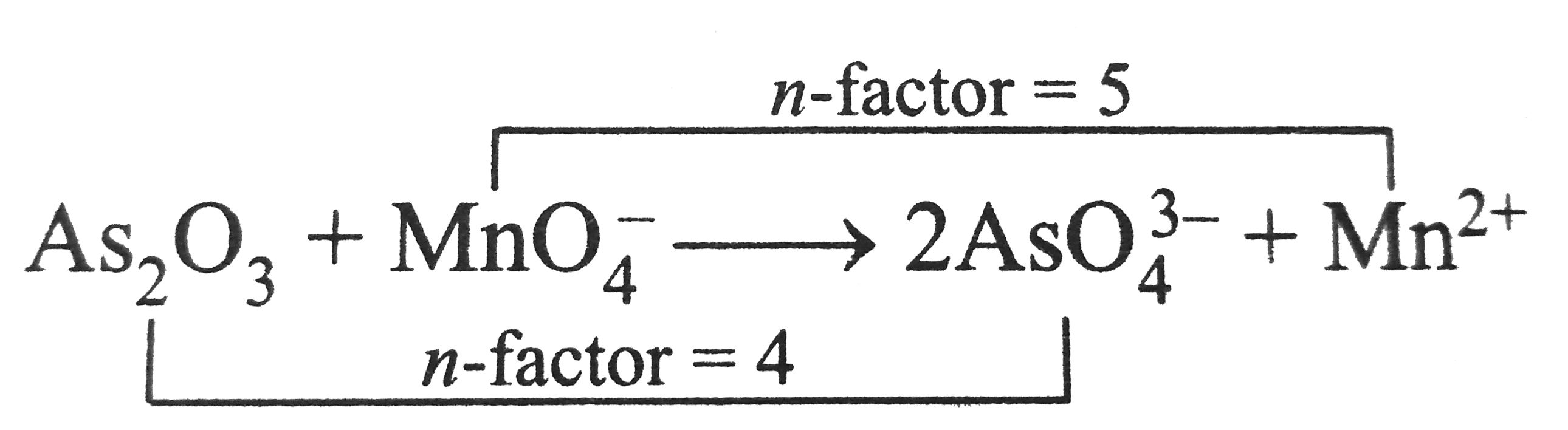A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC CONCEPTS OF CHEMISTRY-Section D - Chapter End Test
- The minimum quantity of H(2)S needed to precipitate 64.5 g of Cu^(2+) ...
Text Solution
|
- 34 g of H(2)O(2) is present in 1120 ml of H(2)O solution. This solutio...
Text Solution
|
- 1.82 g of a metal required 32.5 mL of N-HCl to dissolve it what is the...
Text Solution
|
- What volume at STP of ammonia gas will be required to be passed into 3...
Text Solution
|
- How many grams of NaHSO(3) will be required to react with one litre of...
Text Solution
|
- A 5.0 mL of solution of H(2)O(2) liberates 0.508 g of iodine from acid...
Text Solution
|
- 1.5 litre of a solution of normality N and 2.5 litre of 2 M HCl are m...
Text Solution
|
- The volume of 0.25 MH(3)PO(3) required to neutralise 25 ml of 0.03 M C...
Text Solution
|
- A 0.1097 g sample of As(2)O(3) requried 26.10 mL of KMnO(4) solution f...
Text Solution
|
- Borax in water gives B(4)O(7)^(2-)+7H(2)O rarr 4H(3)BO(3)+2OH^(-) ...
Text Solution
|
- 50 mL of a solution containing 1 g each of Na(2)CO(3), NaHCO(3) and Na...
Text Solution
|
- Density of water at room temperature is 1 g/ml. How many molecules are...
Text Solution
|
- How many molecule are present in 12 L of liquid C Cl(4)? The density o...
Text Solution
|
- 13.4 g of a sample of unstable hydrated salt Na(2)SO(4).XH(2)O was fou...
Text Solution
|
- A solution containing Na(2)CO(3) and NaOH requires 300 ml of 0.1 N HCl...
Text Solution
|
- The amount of water that should be added to 500 ml of 0.5 N solution o...
Text Solution
|
- 100 g CaCO(3) reacts with 1 litre 1 N HCl. On completion of reaction h...
Text Solution
|
- Assertion: For calculating the molality or the mole fraction of solute...
Text Solution
|
- Assertion: As mole is the basic chemical unit, the concentration of th...
Text Solution
|
- Assertion: Isomorphous substances from crystals of same shape and can ...
Text Solution
|