A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
A2Z|Exercise Heisenbergs Uncertainity Principle And Debroglie Equation|44 VideosATOMIC STRUCTURE
A2Z|Exercise Quantum Numbers, Orbitial'S Shape, Electronic Configuration|91 VideosATOMIC STRUCTURE
A2Z|Exercise Bohr'S Model|34 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-Hydrogen Spectrum
- What is the change in the orbit radius when the electron in the hydrog...
Text Solution
|
- A certain dye absorbs light of lamda = 4000 Å and then fluresces light...
Text Solution
|
- The number of spectral line that can be possible when electrons in 6^(...
Text Solution
|
- Ionisation potential of hydrogen atom is 13 . 6 eV. Hydrogen atom in ...
Text Solution
|
- The balmer series occurs between the wavelength of [R = 1.0968 xx 10^7...
Text Solution
|
- The radius of hydrogen atom in its ground state is 5.3 xx 10^-11 m. Af...
Text Solution
|
- Which element has a hydrogen like spectrum whose lines have wavelength...
Text Solution
|
- The frequency corresponding to transition n = 1 to n = 2 in hydrogen a...
Text Solution
|
- The frequency of light emitted for the transition n = 4 to n =2 of He^...
Text Solution
|
- If the wavelength of the first line of the Balmer series of hydrogen ...
Text Solution
|
- The wave number of the first line of Balmer series of hydrogen is 15...
Text Solution
|
- In hydrogen spectrum, the series of lines appearing in ultra violet r...
Text Solution
|
- Which of the following series of transitions in the spectrum of hydrog...
Text Solution
|
- To which electronic transtion between Bohr orbits in hydrogen, the sec...
Text Solution
|
- A photons was absorbed by a hydrogen atom in its ground state, and the...
Text Solution
|
- The energy of hydrogen atom in its ground state is -13.6 eV. The ener...
Text Solution
|
- No. of visible lines when an electron returns from 5^(th) orbit up to ...
Text Solution
|
- Suppose that a hypothetical atom gives a red, green, blue and violet l...
Text Solution
|
- The angular momentum of an electron in a Bohr's orbit of He^+ is 3.165...
Text Solution
|
- The number of possible line of Paschen series when electron jumps from...
Text Solution
|
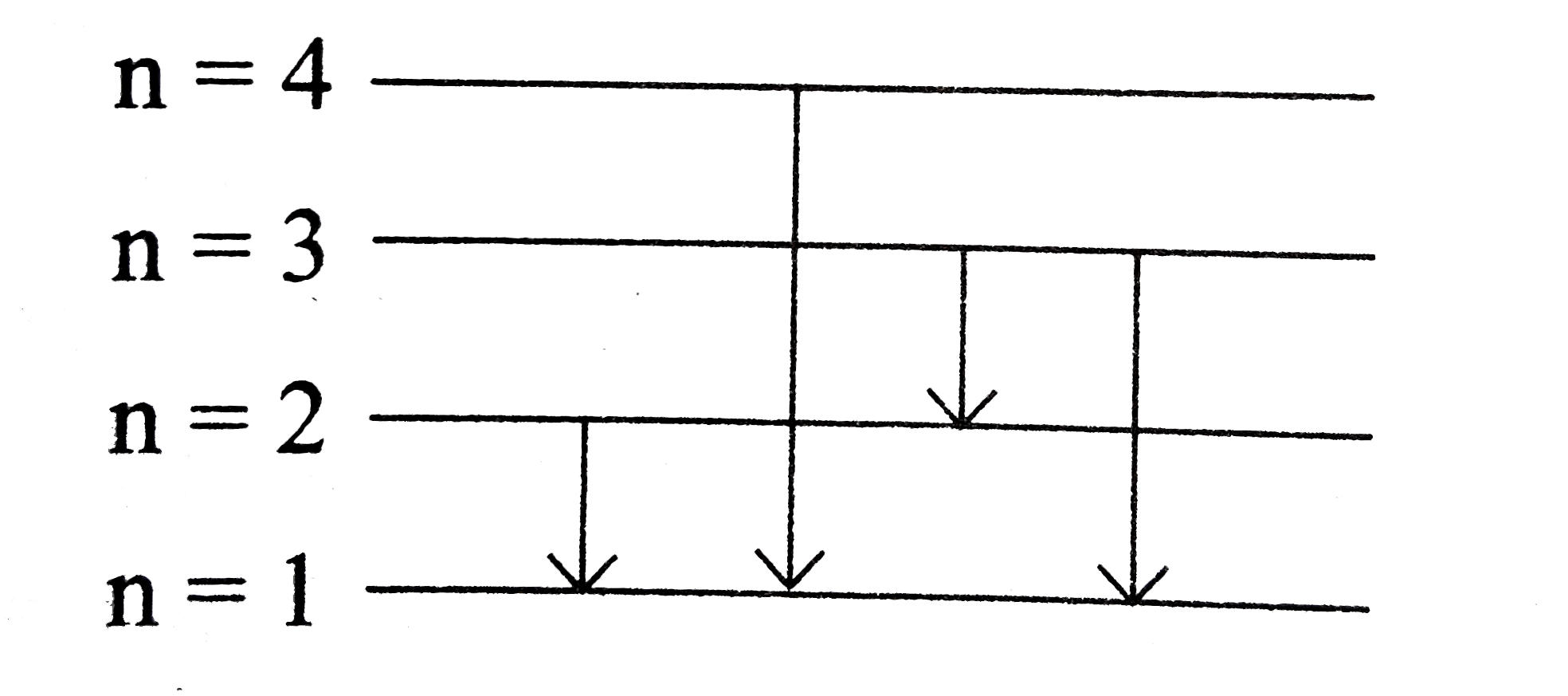 .
.