A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|34 VideosATOMIC STRUCTURE
A2Z|Exercise AIPMT/NEET Questions|59 VideosATOMIC STRUCTURE
A2Z|Exercise Heisenbergs Uncertainity Principle And Debroglie Equation|44 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-Quantum Numbers, Orbitial'S Shape, Electronic Configuration
- Which set is correct for an electron in 4f -orbitial ?
Text Solution
|
- Which set of quantum number is not consistent with the quatum mechanic...
Text Solution
|
- + and - sign of the lobes of py orbital represents.
Text Solution
|
- The maximum number of electrons present in an orbit. l = 3, is .
Text Solution
|
- In a malti-electrons atom which of the following orbitals deseribed...
Text Solution
|
- Which of the following set of quantum number belongs to highest energy...
Text Solution
|
- An electron has principal quantum number 3. The number of its (i) sub-...
Text Solution
|
- Which of the following statement is correct in relation to the hydroge...
Text Solution
|
- If the quantum number l has a value of 2 what are the permitted valu...
Text Solution
|
- Which electronic configuration is not observing the (n + l) rule.
Text Solution
|
- The number of radial nodes of 3s and 2p orbital are, respectively
Text Solution
|
- The "spin-only" magnetic moment [in units of Bohr magneton, (muB)] or...
Text Solution
|
- The correct set of four quantum number for the valence (outermost) ele...
Text Solution
|
- If a shell is having g sub-shell, which is correct statement about pri...
Text Solution
|
- According to Boohr's theory the angular momentum of an electron in 5th...
Text Solution
|
- Which set of quantum number is not possible for electron un 3^(rd) she...
Text Solution
|
- Identify the incorrect statements
Text Solution
|
- Which of the following set of quantum numbers represents the highest e...
Text Solution
|
- The angle made by angular momentum vector of an electron with Z-axis i...
Text Solution
|
- Which of the following orbitals are symmetric about the y-axis ?
Text Solution
|
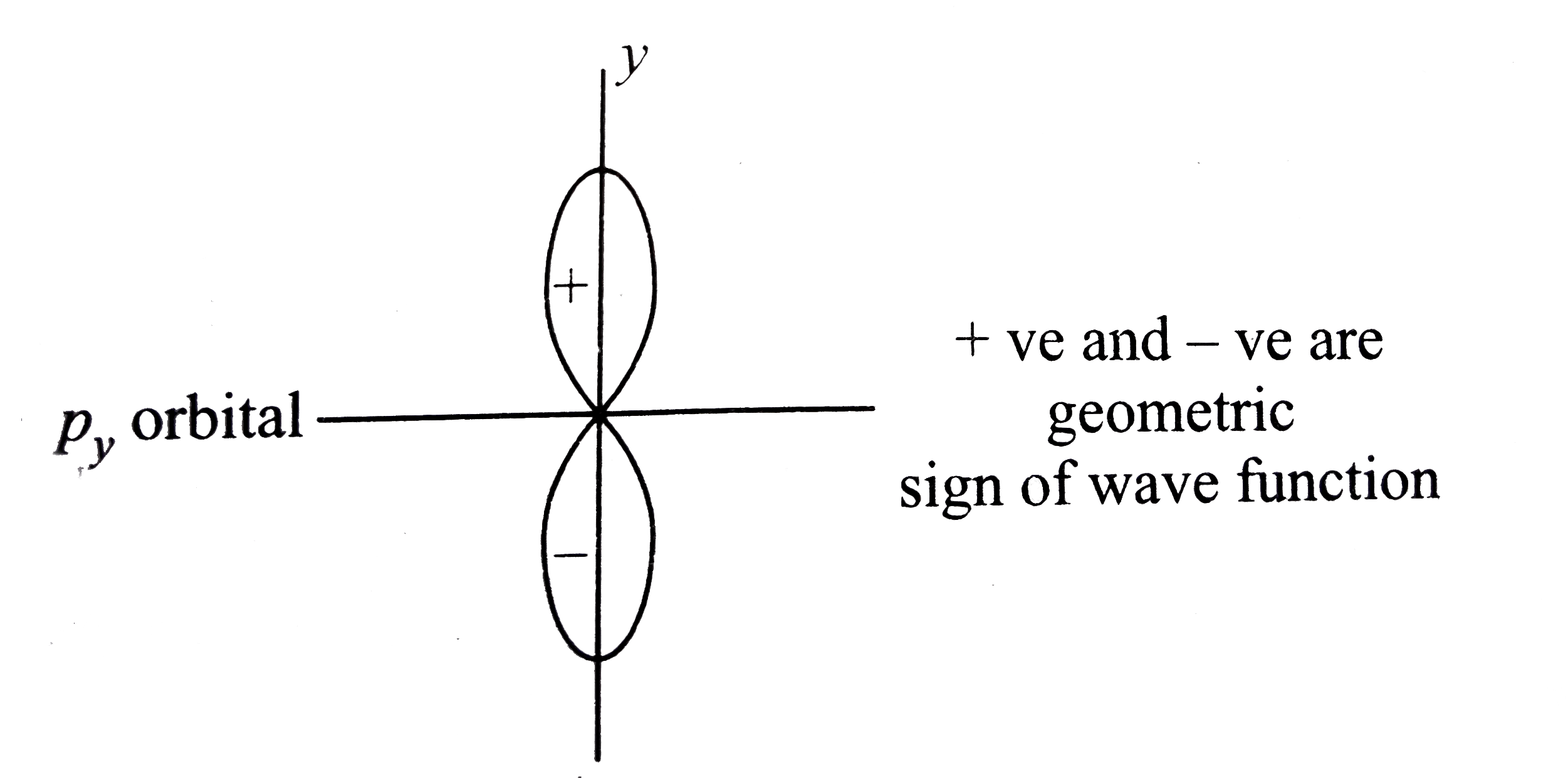 .
.