A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|34 VideosATOMIC STRUCTURE
A2Z|Exercise AIPMT/NEET Questions|59 VideosATOMIC STRUCTURE
A2Z|Exercise Heisenbergs Uncertainity Principle And Debroglie Equation|44 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-Quantum Numbers, Orbitial'S Shape, Electronic Configuration
- The angle made by angular momentum vector of an electron with Z-axis i...
Text Solution
|
- Which of the following orbitals are symmetric about the y-axis ?
Text Solution
|
- The total spin resulting from a d^7 configuration is :
Text Solution
|
- Magnetic moment of V(Z = 23),Cr(Z = 24),and Mn(Z= 25) are x,y,z resp...
Text Solution
|
- The valence shell electronic configuration of the Fe^(2 +) is.
Text Solution
|
- The number of d electrons in Fe^(2+) (atomic number of Fe = 26) is not...
Text Solution
|
- The electrons, identified by quantum number n and l can be placed in t...
Text Solution
|
- Which of the following electronic configuration is not possible accord...
Text Solution
|
- The four quantum number of the valence electron of potassium are.
Text Solution
|
- The configuration 1 s^2, 2 s^2 2 p^5, 3 s^1 shows
Text Solution
|
- For principle quantum number n = 4 the total number of orbitals having...
Text Solution
|
- Cu^(2+) will have the following electronic configuration.
Text Solution
|
- The electronic configuration of an element with atomic number7 i.e. ni...
Text Solution
|
- A gas absorbs a photon of 355 nm and emits at two wavelengths . If on...
Text Solution
|
- Which electronic configuration for oxygen is correct according to Hund...
Text Solution
|
- Which one is the correct outer configuration of chromium.
Text Solution
|
- The electronic configuration of silver atom in ground state is.
Text Solution
|
- Among V(Z = 23), Cr(Z = 24), Mn(Z = 25) which will have highest magnet...
Text Solution
|
- The number of electrons having l = 0 chlorine atom (Z = 17) is
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
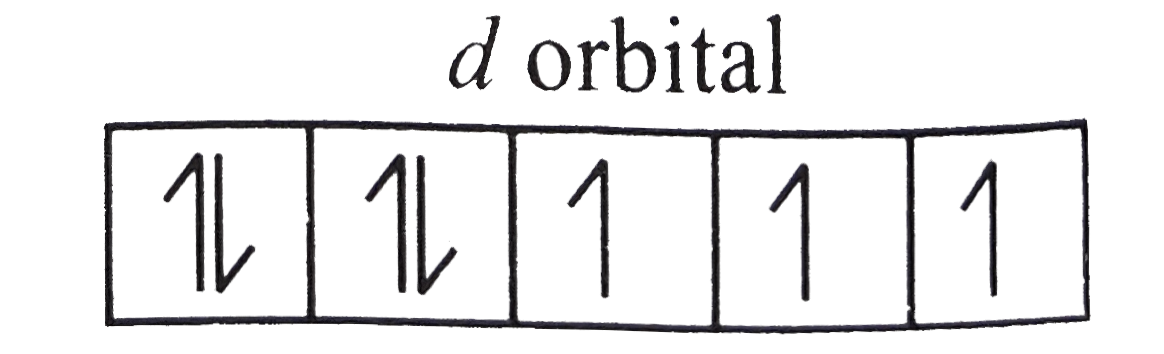 . In other words, there will be `3` unpaired electrons of a `3//2` spin.
. In other words, there will be `3` unpaired electrons of a `3//2` spin.