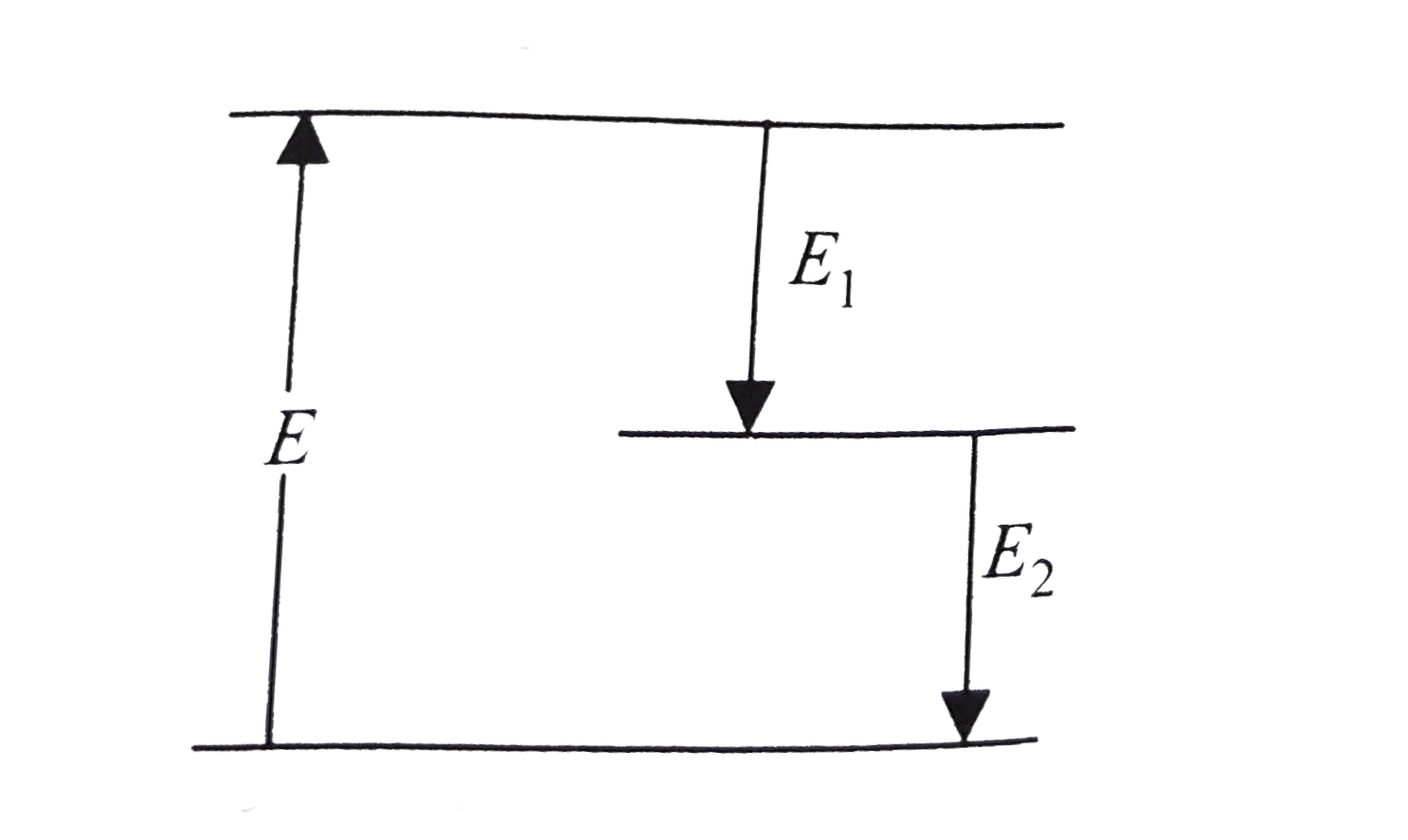
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|34 VideosATOMIC STRUCTURE
A2Z|Exercise AIPMT/NEET Questions|59 VideosATOMIC STRUCTURE
A2Z|Exercise Heisenbergs Uncertainity Principle And Debroglie Equation|44 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-Quantum Numbers, Orbitial'S Shape, Electronic Configuration
- Cu^(2+) will have the following electronic configuration.
Text Solution
|
- The electronic configuration of an element with atomic number7 i.e. ni...
Text Solution
|
- A gas absorbs a photon of 355 nm and emits at two wavelengths . If on...
Text Solution
|
- Which electronic configuration for oxygen is correct according to Hund...
Text Solution
|
- Which one is the correct outer configuration of chromium.
Text Solution
|
- The electronic configuration of silver atom in ground state is.
Text Solution
|
- Among V(Z = 23), Cr(Z = 24), Mn(Z = 25) which will have highest magnet...
Text Solution
|
- The number of electrons having l = 0 chlorine atom (Z = 17) is
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is false? a.The energy of an electron in an or...
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- For s-orbitals, since (Psi orbitals wave function) is independent of a...
Text Solution
|
- With increasing member, the energy difference between adjacent levels ...
Text Solution
|
- How many electrons can fit into the orbitals that comprise the 3^(rd) ...
Text Solution
|
- Which of the following statements concerning the four quantum numbers ...
Text Solution
|
- The possible value of l and m for the last electron in the Cl^(- )ion ...
Text Solution
|
- dz^2 orbital has :
Text Solution
|