A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|34 VideosATOMIC STRUCTURE
A2Z|Exercise AIPMT/NEET Questions|59 VideosATOMIC STRUCTURE
A2Z|Exercise Heisenbergs Uncertainity Principle And Debroglie Equation|44 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-Quantum Numbers, Orbitial'S Shape, Electronic Configuration
- dz^2 orbital has :
Text Solution
|
- The managanese (Z = 25) has the outer configuration.
Text Solution
|
- If the electronic structure of oxygen atom is written as 1s^2, 2 s^2 ...
Text Solution
|
- A given orbital is labelled by the magnetic quantum number m = -1. Thi...
Text Solution
|
- The quantum number of obtained from the Schrӫdinger wave length is.
Text Solution
|
- The set of quantum number not applicable to an electron
Text Solution
|
- Maximum numbers of electrons in a subshell is given by-
Text Solution
|
- Which of the following sets of quantum numbers represents an imposs...
Text Solution
|
- Which of the following statements about nodal planes is/are not true.
Text Solution
|
- Which element is represented by the following electronic configuration...
Text Solution
|
- For the energy level in an atom which one of the following statement i...
Text Solution
|
- The electronic configurations of Cr^24 and Cu^29 are abnormal -
Text Solution
|
- The below configuration is not correct as it violates .
Text Solution
|
- The maximum probability of finding electron in the d(xy) orbital is -
Text Solution
|
- In centre-symmetrical system, the orbital angular momentum, a measure ...
Text Solution
|
- The orbital angular momentum of an electron in2sorbital is
Text Solution
|
- What are the values of the orbital angular momentum of an electron in ...
Text Solution
|
- The z-component of angular momentum of an electron in an atomic o...
Text Solution
|
- Which of the following symbols represent an atomic orbital ?
Text Solution
|
- Which orbitals is non-directional ?
Text Solution
|
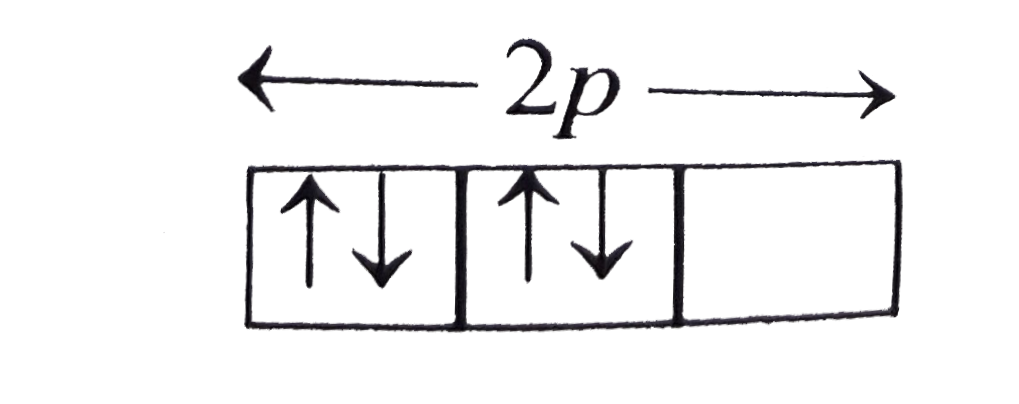 `1s^2, 2 s^2` it would violate-
`1s^2, 2 s^2` it would violate-