A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC STRUCTURE-AIIMS Questions
- Azimuthal quantum number defines.
Text Solution
|
- For principle quantum number n = 4 the total number of orbitals having...
Text Solution
|
- The maximum number of electrons that can be accommodated in an orbital...
Text Solution
|
- Number of unpaired electrons in 1 s^2 2 s^2 2 p^3 is.
Text Solution
|
- For the energy levels in an atom , which of the following statement ...
Text Solution
|
- The statements. (i) In filling a group of orbitals of equal energy, ...
Text Solution
|
- Energy of atomic orbitals in a particular shell is in the order.
Text Solution
|
- Which of the following explains the sequence of filling the electrons ...
Text Solution
|
- Which of the following arrangements of electron is mostly likely to th...
Text Solution
|
- Wavelength of particular transition for H atom is 400 nm. What can be ...
Text Solution
|
- The possible number of orientations of a sun-shell is (2 l + 1) The ...
Text Solution
|
- Humphry series discovered in H-"atomic" spectra has lowest energy rad...
Text Solution
|
- Statement : Aufbau rule is violated in writing electronic configuratio...
Text Solution
|
- A resonance hybrid is always more stable than any of its canonical str...
Text Solution
|
- Cathode rays do not travel in straight lines. Cathode rays do not pe...
Text Solution
|
- Electrons revolving around the nucleus do not fall into the nucleus be...
Text Solution
|
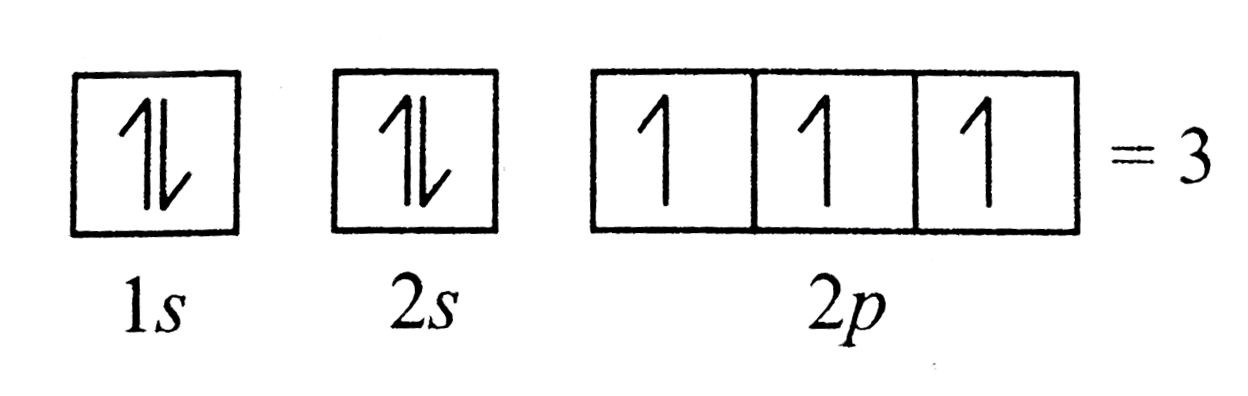 .
.