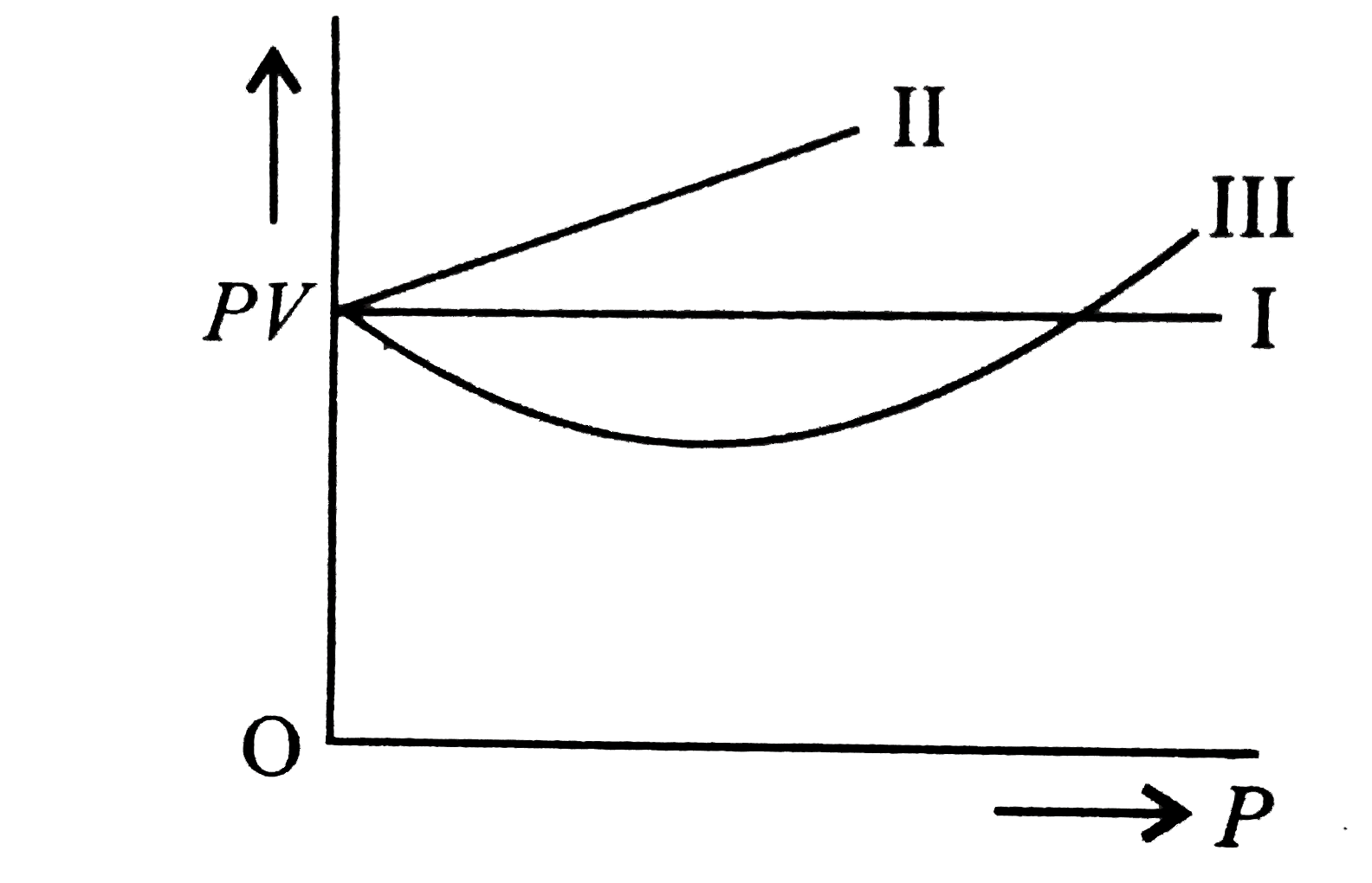
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
A2Z|Exercise Liquid State|17 VideosSTATES OF MATTER
A2Z|Exercise Section B - Assertion Reasoning|23 VideosSTATES OF MATTER
A2Z|Exercise Kinectic Theory Of Gases, Maxwell Distribution Of Speed|45 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|60 Videos
Similar Questions
Explore conceptually related problems
A2Z-STATES OF MATTER-Van Der Waals Gas Equation And Liquefaction Of Gases
- At low pressure, the van der Waals equation is reduced to
Text Solution
|
- The temperature at which a real gas obeys the ideal gas laws over a wi...
Text Solution
|
- Actual graph for the given parameters in (Q.25) will be
Text Solution
|
- A real gas at a very pressure occupies
Text Solution
|
- Calculate the compressibility factor for CO(2) if one mole of it occup...
Text Solution
|
- For the non-zero value of the force of attraction between gas molecule...
Text Solution
|
- The compressibility of a gas is less than unity at STP, therefore,
Text Solution
|
- Compressibility factor for H(2) behaving as real gas is:
Text Solution
|
- The value of compressibility factor at the critical state the gas matc...
Text Solution
|
- Pressure exerted by 1 mole of methane, in a 0.25 litre container at 30...
Text Solution
|
- If bar(V) is the observed molor volume of real gas and bar(V)(id) is t...
Text Solution
|
- Which of the following is the correct set of volume calculated by idea...
Text Solution
|
- A gas can be liquefied by pressure alone when its temperature
Text Solution
|
- Regarding the van der Waals constant which of the following is/are cor...
Text Solution
|
- Which of the following satisfies the greater compressibility of real g...
Text Solution
|
- The correct order of normal boiling of O(2), N(2), NH(3) and CH(4) for...
Text Solution
|
- Which of the following characterstics the critical point?
Text Solution
|
- A real gas most closely approaches the behaviour of an ideal gas at:
Text Solution
|
- Which of the following is true at the critical point?
Text Solution
|
- Which of the given sets of temperature and pressure will cause a gas t...
Text Solution
|