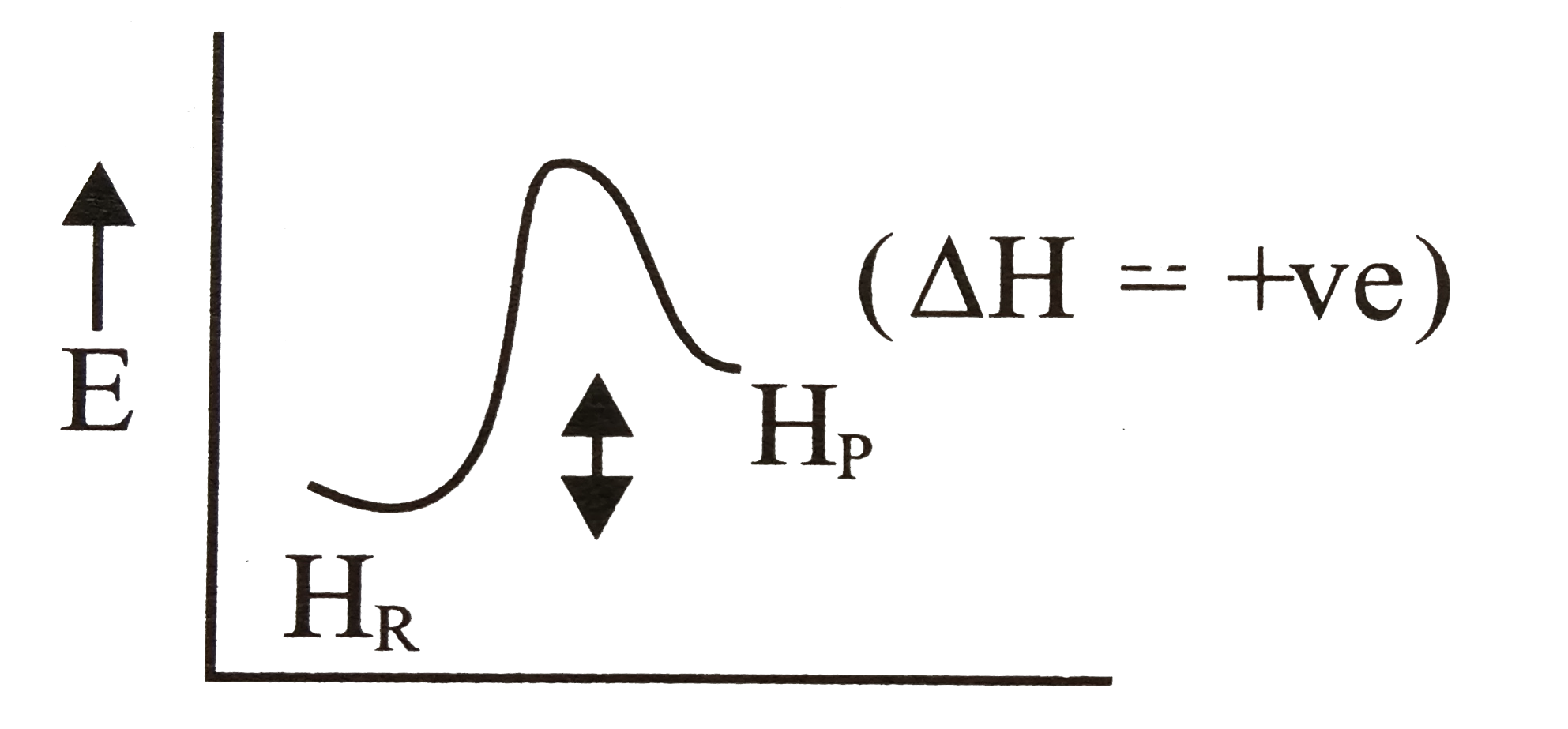A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
A2Z|Exercise Entropy, Gibb'S Energy And Spontaneity Of Process|52 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Heat Capacity And Calorimetry|13 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL THERMODYNAMICS-Work, Internal Energy And Enthalpy
- Given that 3C(s)+2Fe(2)O(3)(s)rarr4Fe(s)+3CO(2)(g), DeltaH^(@)=-93...
Text Solution
|
- For which of the following equations is the enthapy change at 25^(@)C ...
Text Solution
|
- For an endothermic reaction,
Text Solution
|
- 4.8 g of C (diamond) on complete combustion evolves1584 kJ of heat. Th...
Text Solution
|
- In the reaction CO(2)(g)+H(2)(g)rarrCO(g)+H(2)O(g), DeltaH=2.8 kJ ...
Text Solution
|
- For the reaction A(g)+2B(g)rarr2C(g)+3D(g), the value of DeltaH at...
Text Solution
|
- For the reaction N(2)(g) + 3H(2)(g) hArr 2NH(3)(g), DeltaH=?
Text Solution
|
- If C(s)+O(2)(g)rarrCO(2)(g),DeltaH=X and CO(g)+1//2O(2)(g)rarrCO(2)(g)...
Text Solution
|
- Heat of solution of BaCl(2).2H(2)O=200 kJ mol^(-1) Heat of hydration...
Text Solution
|
- The mutual heat of neutralisation of 40 g NaOH and 60CH(3)COOH will be
Text Solution
|
- DeltaH(comb)^(@) of carbon is -x kJ mol^(-1). The standard formation o...
Text Solution
|
- The heat of combustion of carbon is 394 kJ. The heat evolved in combus...
Text Solution
|
- Heat of neutralisation of a strong acid by a strong base is equal to D...
Text Solution
|
- Heat of neutralisation is least when
Text Solution
|
- The standard heat of formation of U(3)O(8) is -853.5 kcl//mol and the ...
Text Solution
|
- Given N(2)(g)+3H(g)=2NH(3)(g),DeltaH^(@)=22 kcal. The standard enthalp...
Text Solution
|
- DeltaH for CaCO(3)(s)rarrCaO(s)+CO(2)(g) is 176 kJ mol^(-1) at 1240 K....
Text Solution
|
- C("diamond")+O(2)(g)rarrCO(2)(g),DeltaH=-395 kJ ......(i) C("graphit...
Text Solution
|
- Given that O(g)+e^(-)rarrO^(-)(g) , DeltaH=34Kcal mol^(-1) O(g)+2e...
Text Solution
|
- The enthalpies of combustion of carbon and carbon momoxide are -390KJ ...
Text Solution
|