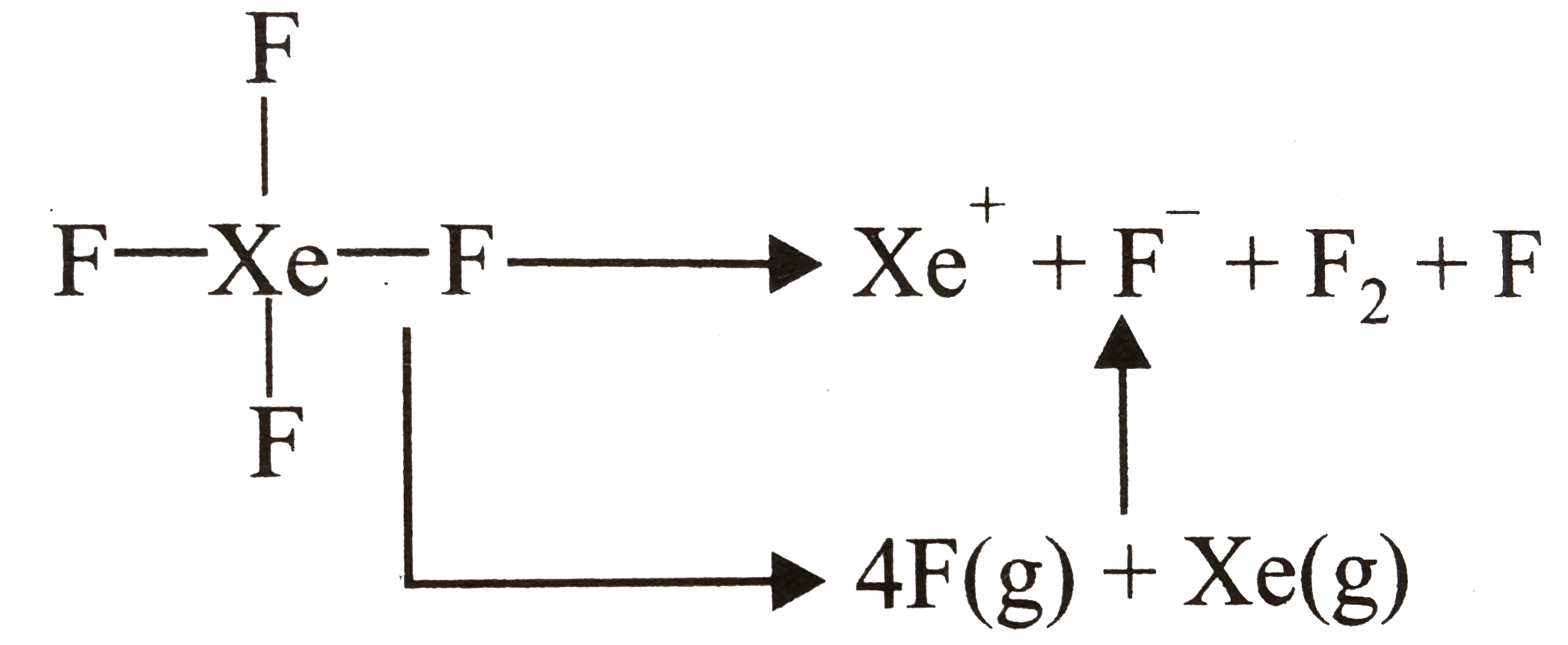A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
A2Z|Exercise Entropy, Gibb'S Energy And Spontaneity Of Process|52 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Heat Capacity And Calorimetry|13 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL THERMODYNAMICS-Work, Internal Energy And Enthalpy
- Heat of hydrogenation of ethene is x(1) and that of benzene is x(2) . ...
Text Solution
|
- Under the same conditions, how many mL of 1M KOH and 0.5M H(2)SO(4)sol...
Text Solution
|
- Bond energy of (N-H) bond is yKJ mol^(-1) under standard state. Thus,c...
Text Solution
|
- The enthaplpy changes state for the following processes are listed bel...
Text Solution
|
- For the equations C1 ("diamond") +2H(2)(g)rarrCH(4)(g),DeltaH(1) C...
Text Solution
|
- If H(2)(g)=2H(g),DeltaH=104cal , then heat of atomisation of hydrogen ...
Text Solution
|
- The average Xe-F bond energy is 34Kcal//mol , first I.E. Of Xe is 279K...
Text Solution
|
- The standard enthalpy of formation of NH(3) is -46.0KJ mol^(-1) . If t...
Text Solution
|
- Given H(2)(g)=2H(g)Delta(H-H)=103Kcal mol^(-1) The heat of reaction ...
Text Solution
|
- Energy required to dissociate 4g of gaseous hydrogen into free gaseous...
Text Solution
|
- The H-H bond energy is 430KJ mol^(-1) and Cl-Cl bonds is 240KJ mol^(-1...
Text Solution
|
- Given the bond energies N=N , H and H-H bond are 945, 436 and 391KJmol...
Text Solution
|
- AB , A(2) and B(2) are diatomic molecules, if the bond enthalpies of A...
Text Solution
|
- The standard enthalpies of formation of SF(6)(g) , S(g) and F(g) are -...
Text Solution
|
- Using bond enthalpies (symbolized by epsilon) an estimated value of De...
Text Solution
|
- The heat of formation of CO(2) is -407KJ//mol . The energy required fo...
Text Solution
|
- Heat evolved in the reaction H(2)+Cl(2) rarr 2HCl is 182 kJ Bond ene...
Text Solution
|
- Enthalpy of formation of 2mol of NH(3)(g) is -90KJ , and DeltaH(H-H) a...
Text Solution
|
- Oxidising power of chlorine in aqueous solution can be determined by t...
Text Solution
|
- The value of DeltaH(O-H) is 109Kcal//mol . The enthalpy of formation o...
Text Solution
|