A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
A2Z|Exercise Calculation Of Equilibrium Constant|20 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Application Of Equllibrium Constant (K)|65 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL EQUILIBRIUM-Section D - Chapter End Test
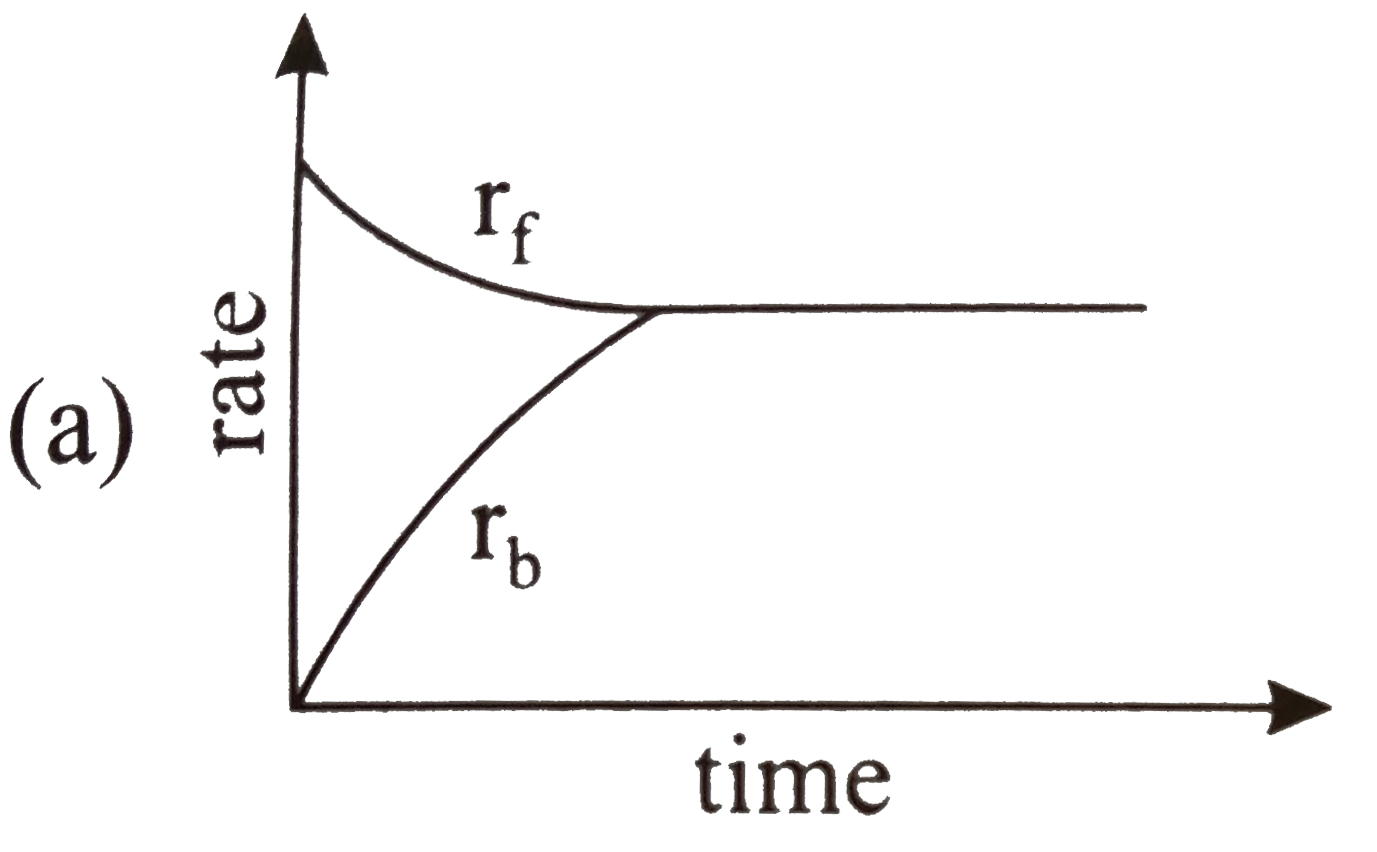
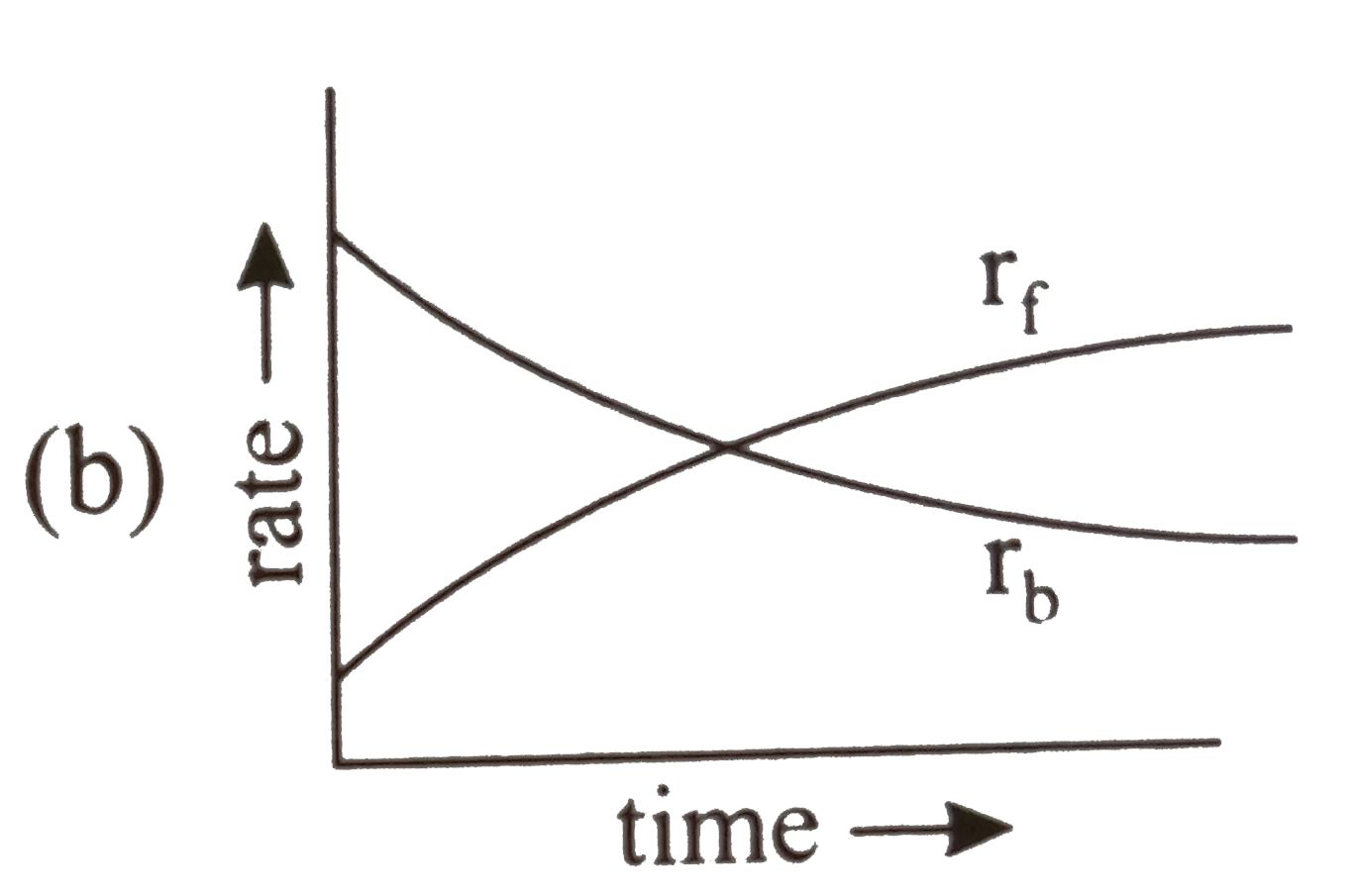
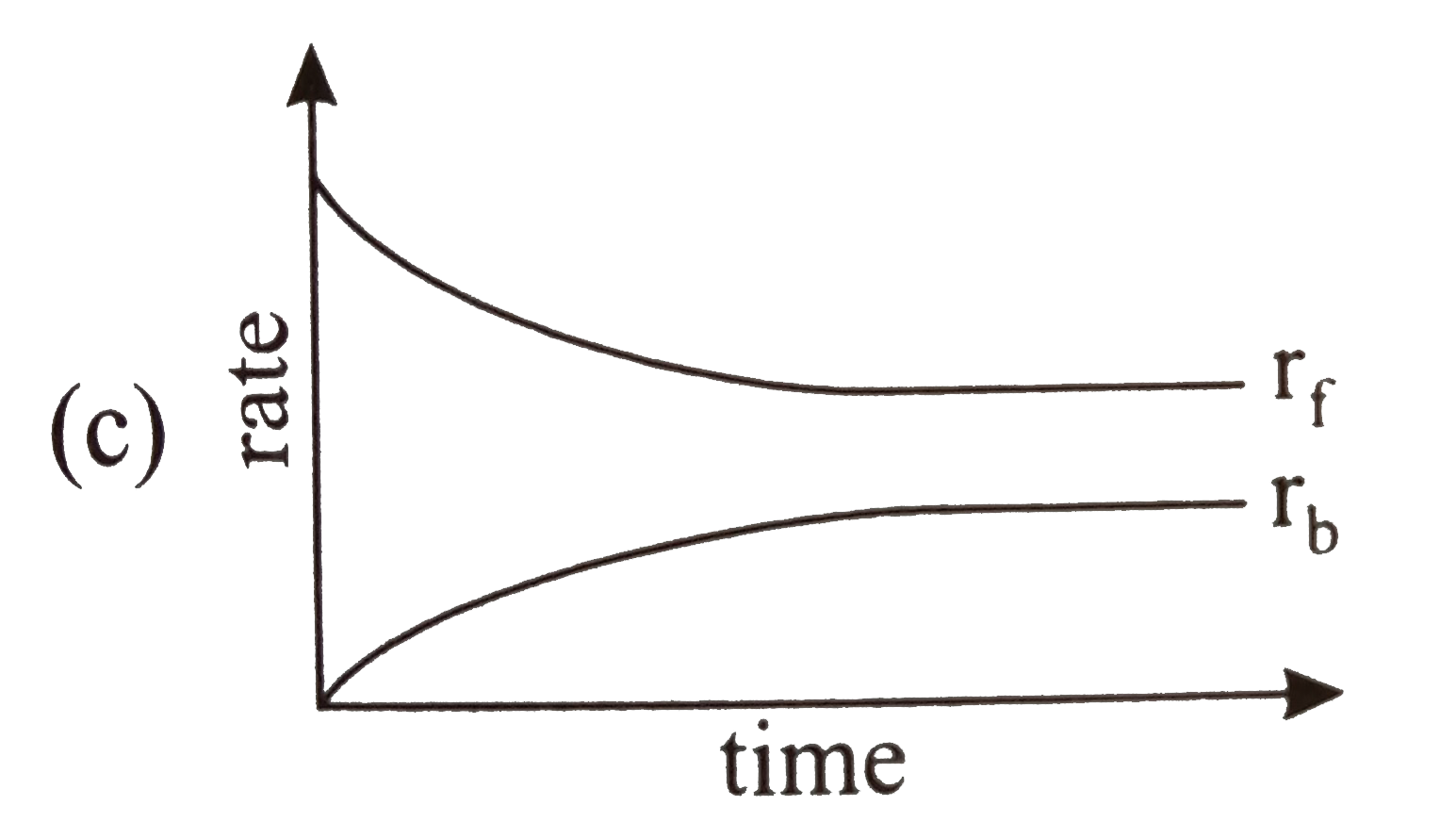
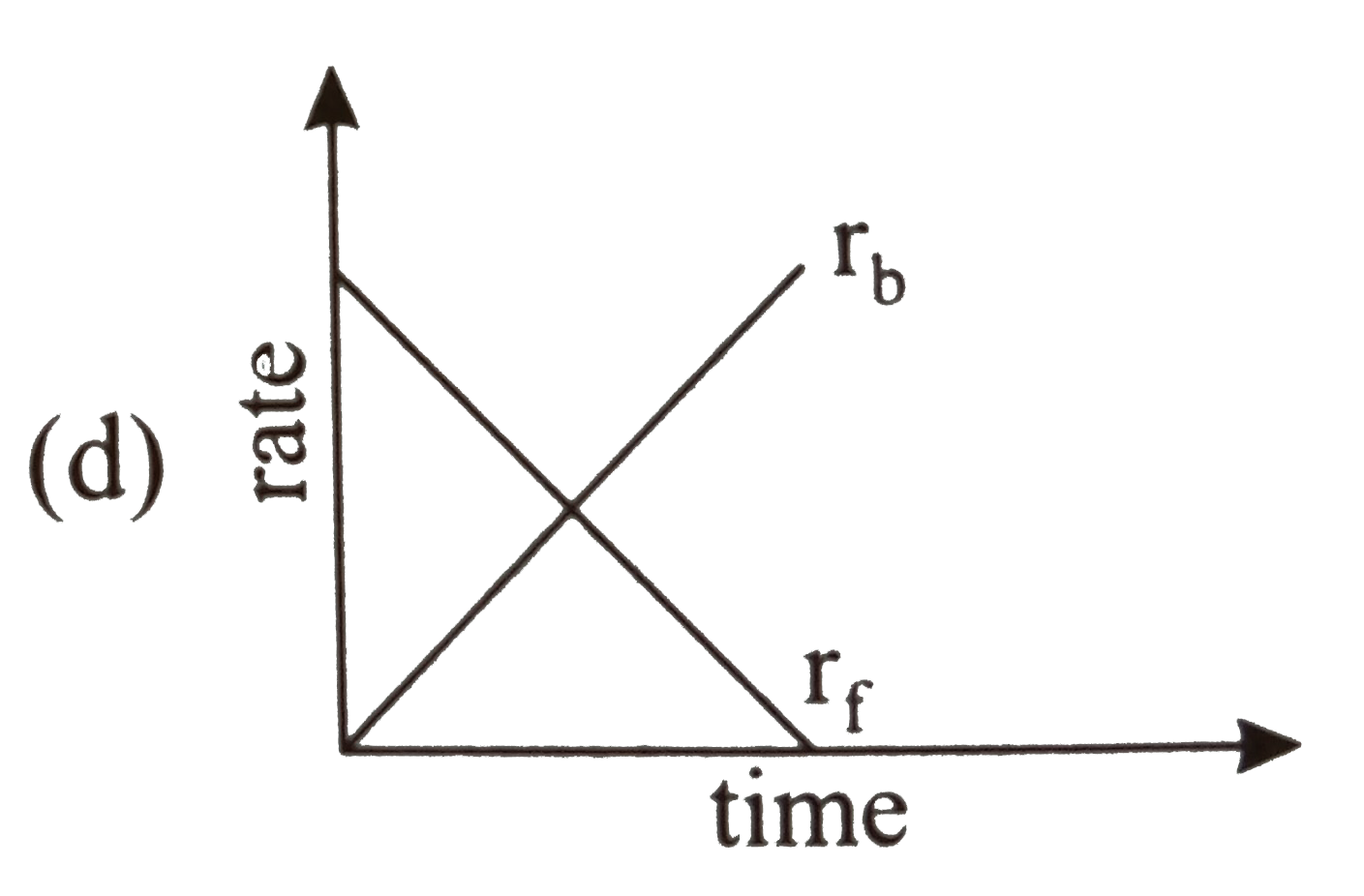
- Rate of reaction curve for equilibrium can be like: [r(f) = forward ra...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delt...
Text Solution
|
- In which of the following equilibrium, the value of K(p) is less than ...
Text Solution
|
- At 298 K equilibrium constant K(1) and K(2) of following reaction SO(2...
Text Solution
|
- If DeltaG^(@) for the reaction given below is 1.7 kJ, the equilibrium ...
Text Solution
|
- Calculate DeltaG^(Theta) for the conversion of oxygen to ozone, ((3)/(...
Text Solution
|
- Given reaction is 2X((gas)) + Y((gas))hArr2Z((gas)) + 80 Kcal Which ...
Text Solution
|
- Consider the reaction HCN((aq))hArrH((aq))^(+) + CN((aq))^(-) . At equ...
Text Solution
|
- In which of the following equilibrium system the rate of the backward ...
Text Solution
|
- For which of the following K(p) may be equal to 0.5 atm
Text Solution
|
- The vapour density of undecomposed N(2)O(4) is 46. When heated, vapour...
Text Solution
|
- If pressure is applied to the following equilibrium, liquid hArr vapou...
Text Solution
|
- For the reaction, A+BhArr3C, at 25^(@)C, a 3 litre vessel contains 1, ...
Text Solution
|
- The equilibrium constant for a reacton N(2)(g)+O(2)(g)=2NO(g) is 4xx...
Text Solution
|
- In the system A((s))hArr2B((g))+3C((g)), if the concentration of C at ...
Text Solution
|
- In a reaction at equilibrium, 'x' mole of reactant A decompose to give...
Text Solution
|
- If CuSO(4).5H(2)O((s))hArrCuSO(4).3H(2)O((s)) + 2H(2)O((l)) K(p) = 1.0...
Text Solution
|
- In the system, LaCI(3(s)) + H(2)O(g) + heat rarr LaCIO(s) + 2HCI(g) , ...
Text Solution
|
- The equilibrium constant for the reaction N(2)(g)+O(2)(g) hArr 2NO(g) ...
Text Solution
|
- For the decomposition reaction: NH(2)COONH(4(s))hArr2NH(3(g))+CO(2(g...
Text Solution
|
- For a reaction A((g)) + B((g))hArrC((g)) + D((g)) the intial concentra...
Text Solution
|