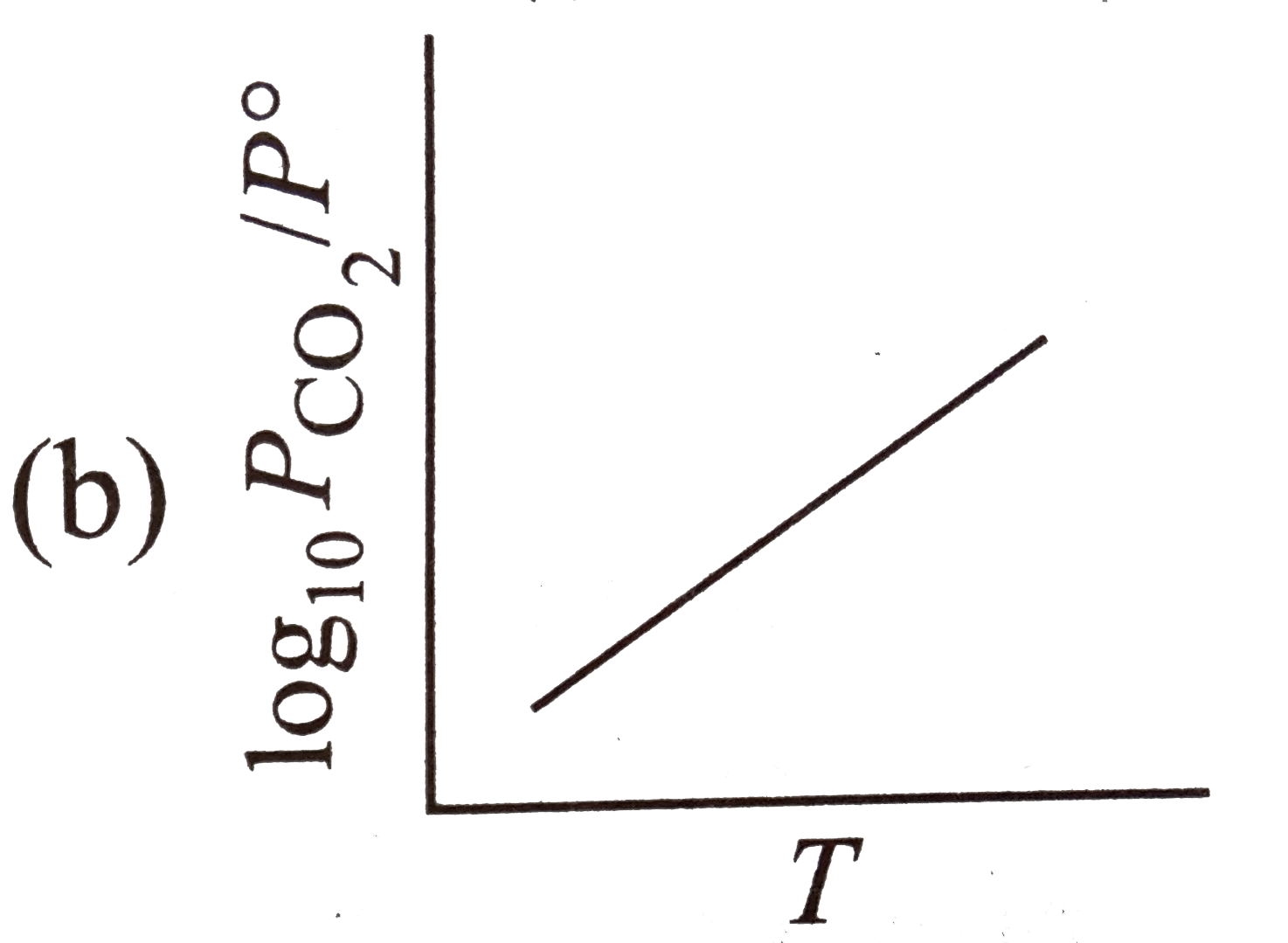
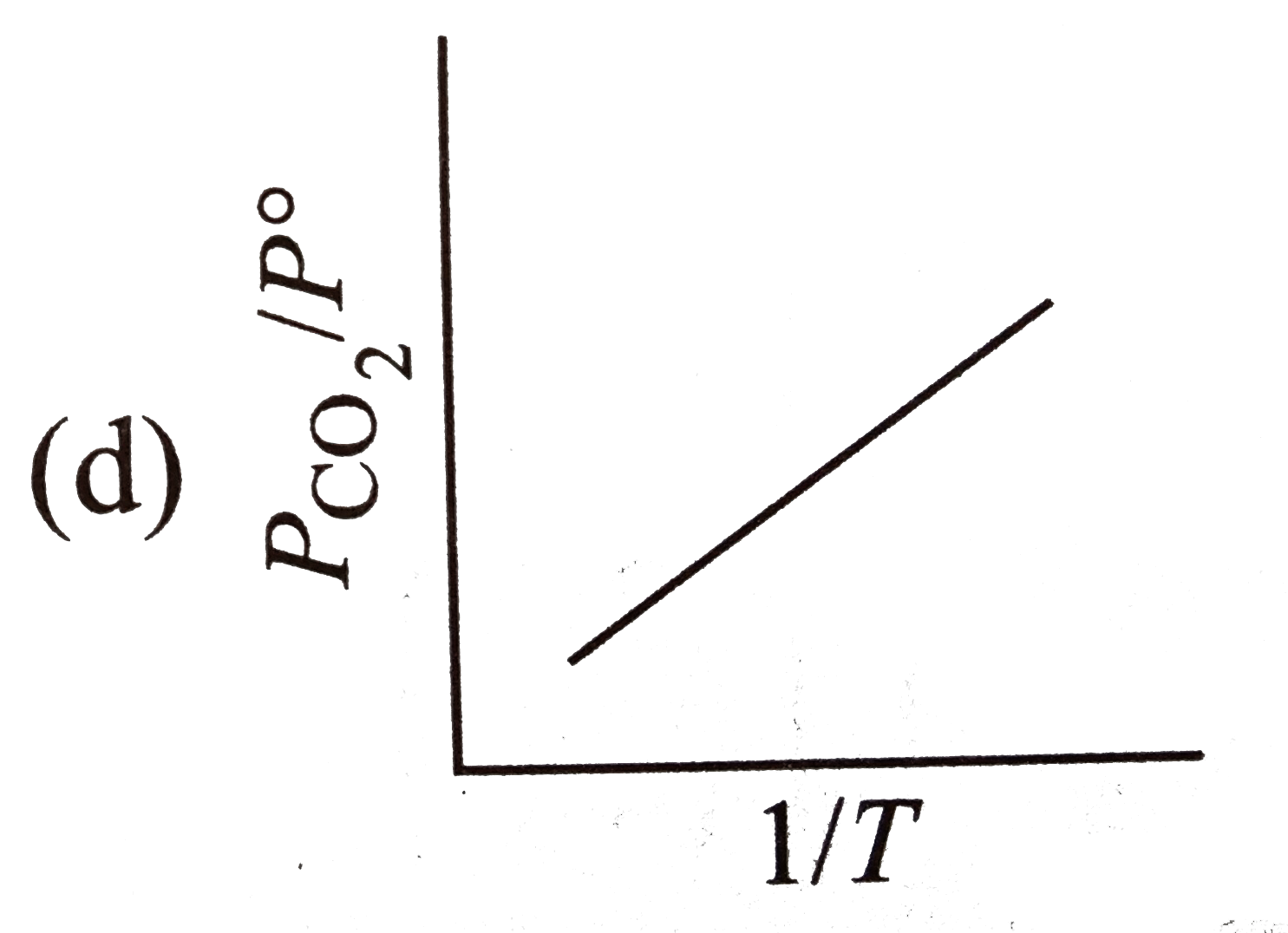
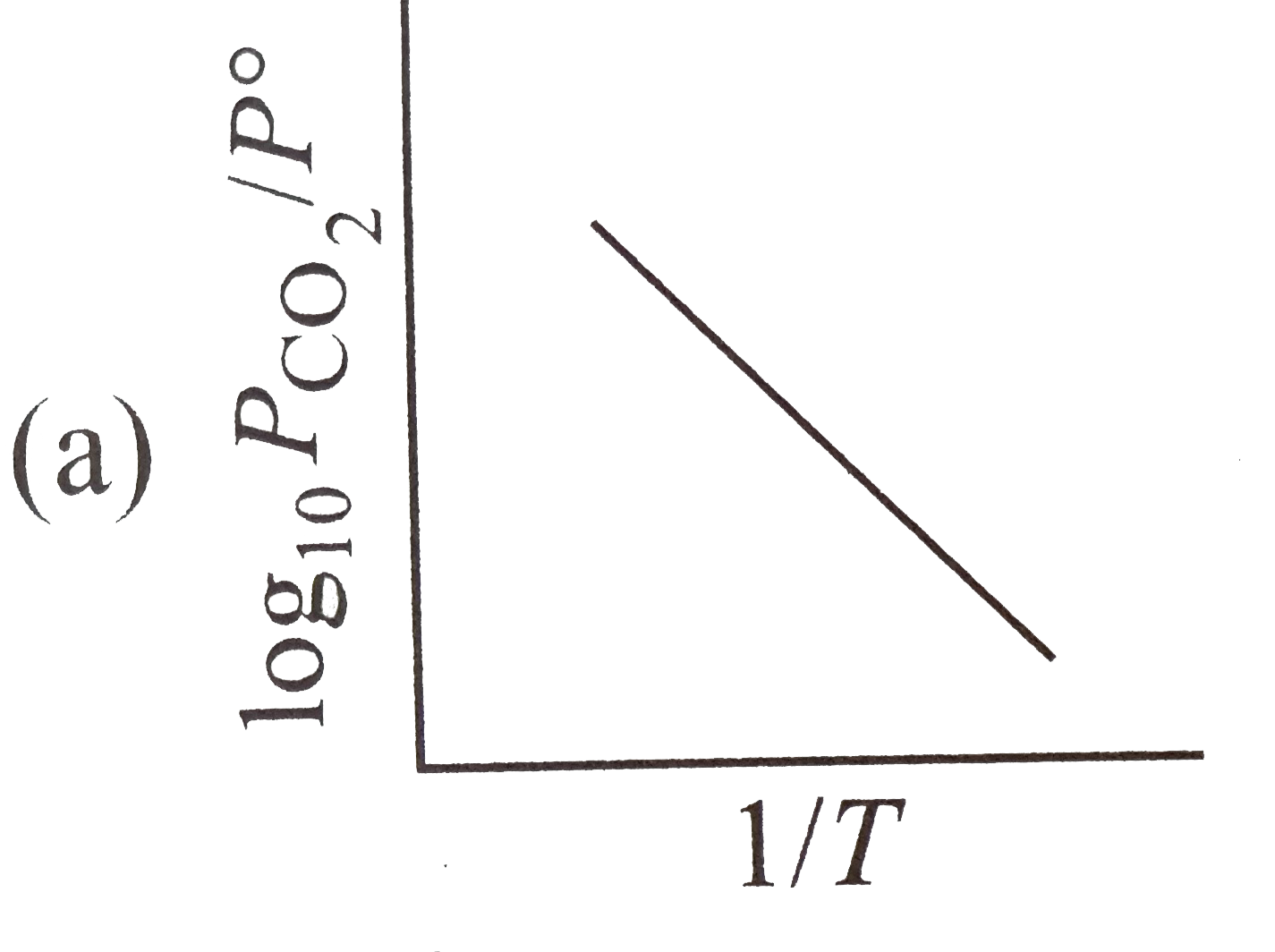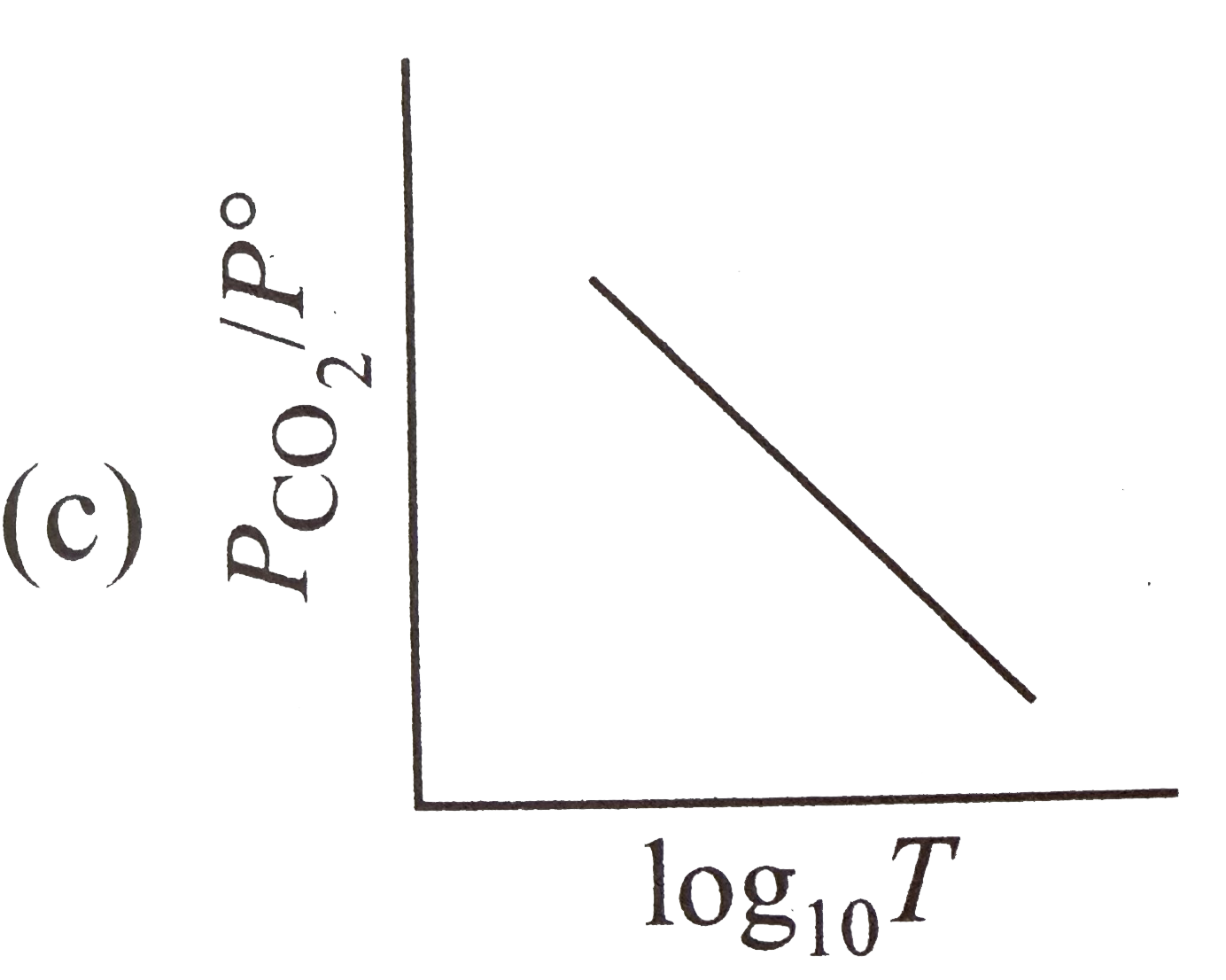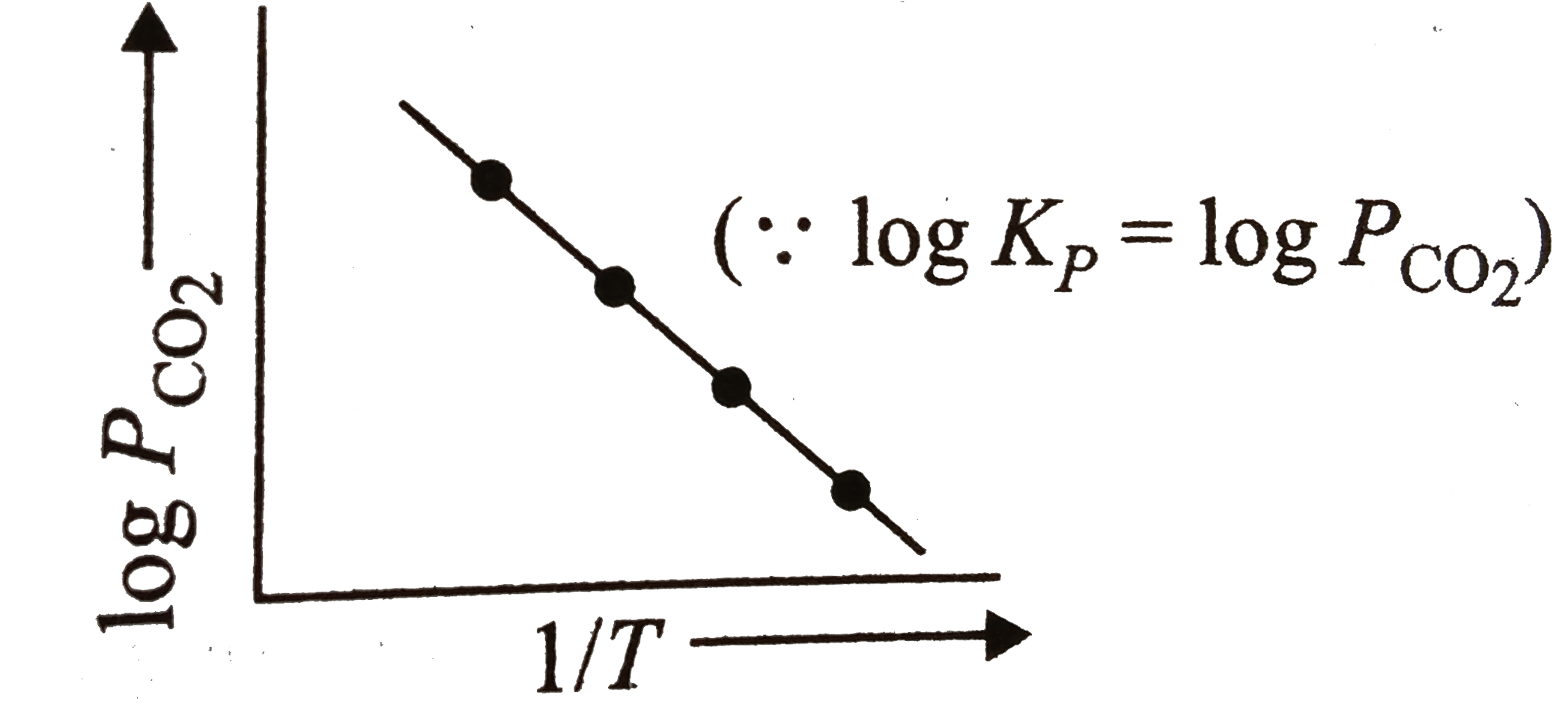A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
A2Z|Exercise Degree Of Dissociation, Vapour Density And Simultaneous Equilibria|20 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section B - Assertion Reasoning|18 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Application Of Equllibrium Constant (K)|65 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL EQUILIBRIUM-Le - Chatellers'S Principle
- The exothermic formation of C1F(3) is represented by the equation C1(2...
Text Solution
|
- In an equilibrium reaction H(2)(g) + I(2)(g)hArr2HI(g) Delta H = - 30...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delt...
Text Solution
|
- Formation of SO(3) take place according to the reaction 2SO(2) + O(2)h...
Text Solution
|
- For the gas phase reaction C(2)H(4)+H(2) hArr C(2)H(6)(DeltaH=-32.7 ...
Text Solution
|
- For the chemical reaction 3X(g)+Y(g) hArr X(3)Y(g), the amount of ...
Text Solution
|
- In an equilibrium reaction for which Delta G^(@) = 0 , the equilibrium...
Text Solution
|
- Consider the following reversible reactionat equilibrium: 2H(2)O(g) ...
Text Solution
|
- In which of the following system, doubling the volume of the container...
Text Solution
|
- In a vessel containing SO(3), SO(2) and O(2) at equilibrium, some heli...
Text Solution
|
- The equilibrium SO(2)Cl(2)(g) hArr SO(2)(g)+Cl(2)(g) is attained at 25...
Text Solution
|
- Delta G^(@)(HI, g) ~= + 1.7 kJ. What is the equilibrium constant at 25...
Text Solution
|
- Which of the following equilibrium will shift to right side on increas...
Text Solution
|
- In a reaction A(2)(g)+4B(2)(g) hArr 2AB(4)(g), DeltaH lt 0. The format...
Text Solution
|
- Sodium sulphate dissolves in water with evolution of heat. Consider a ...
Text Solution
|
- N(2) + 3H(2)hArr2NH(3) If temperature of following equilibrium reactio...
Text Solution
|
- Consider the equilibrium N(2)(g) + 3H(2)(g)hArr2NH(3)(g) , Delta H =...
Text Solution
|
- In the system AB((s))hArrA((g))+B((g)) doubling the quantity of AB((s)...
Text Solution
|
- H(2(g)) + I(2(g))hArr2HI((g)) Delta H = + q cal, the formation of HI:
Text Solution
|
- The standard state Gibbs's energy change for the isomerisation reactio...
Text Solution
|