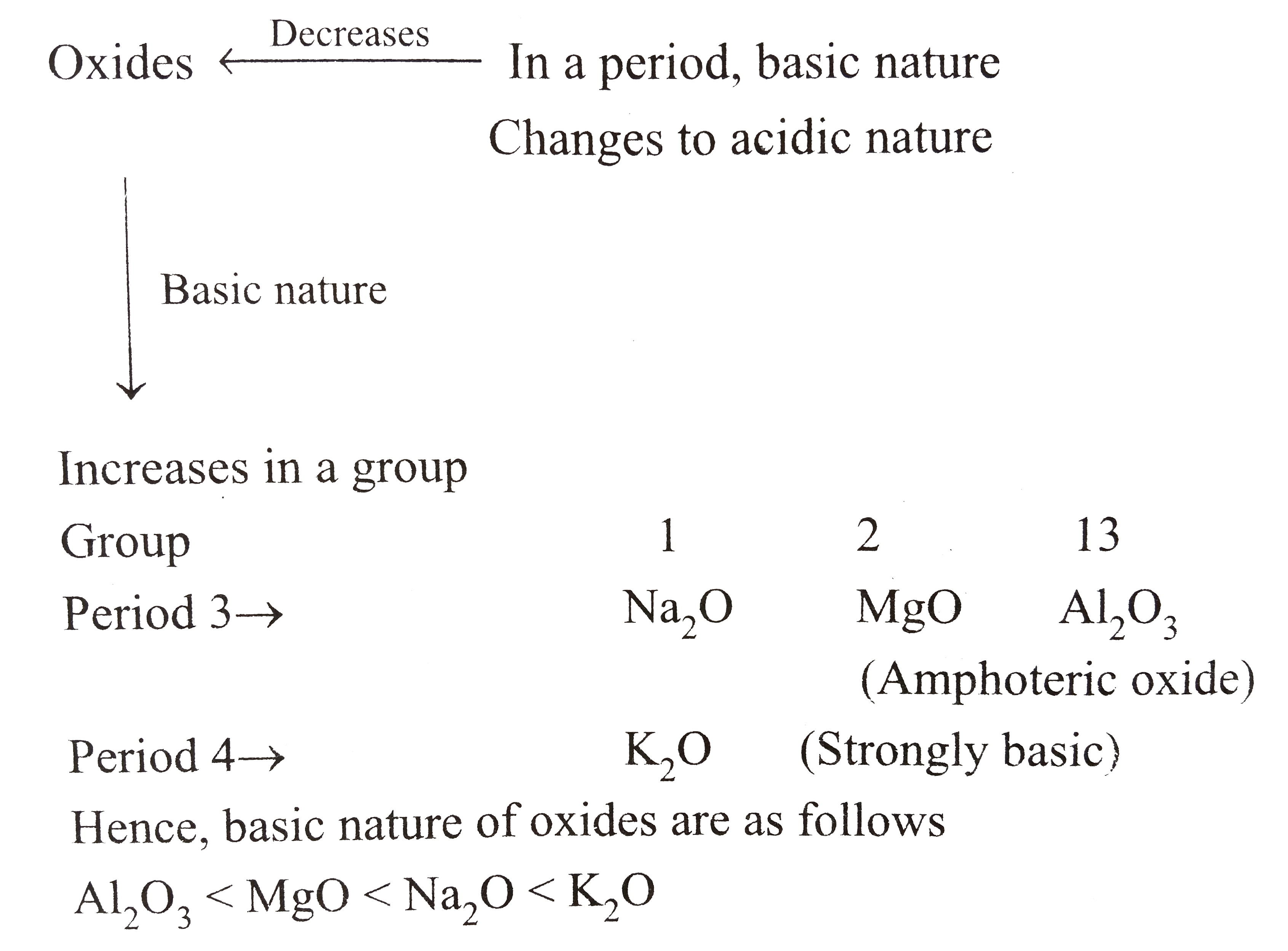A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section B - Assertion Reasoning|22 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise AIPMT/ NEET Questions|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Electron Gain Enthalpy|34 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES-Electronegativity And Nature Of Oxides
- Which of the following set has the strongest tendency to form anions?
Text Solution
|
- 3 and 6 electrons are present in the outermost orbit A and B respectiv...
Text Solution
|
- Keeping in view the peroidic law and the periodic table suggest which...
Text Solution
|
- Increasing order of acid strength of halogen acid is
Text Solution
|
- Which of the following is the most electropositivite element?
Text Solution
|
- The basis of keeping the elements in the group of periodic table is
Text Solution
|
- Which of the following sequence correctly represents the decreasing ac...
Text Solution
|
- which of the following gas does not have an octet or eight electrons i...
Text Solution
|
- Oxidising action increases in halogen in the following order
Text Solution
|
- the maximum valency of an element with atomic number 7 is
Text Solution
|
- which one of the following oxides is neutral?
Text Solution
|
- which of the following is the correct order of gradually decreasing ba...
Text Solution
|
- In which of the following arrangements , the order is not correct acco...
Text Solution
|
- The strongest reducing agent is
Text Solution
|
- The order in which the following oxides are arranged according to decr...
Text Solution
|
- Acidity of pentoxides in VA group
Text Solution
|
- Which of the following statement is correct with respect to the proper...
Text Solution
|
- The ionic mobility of alkali metal ions in aqueous solution is maximum...
Text Solution
|
- The following statements regarding the periodic trends of chemical rea...
Text Solution
|
- Which one of the following orders presents the correct sequence of the...
Text Solution
|