A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-ENVIRONMENTAL CHEMISTRY-Section D - Chapter End Test
- Which of the following is the coldest region of atmosphere
Text Solution
|
- Most hazardous metal pollutant of automobile exhausts is :
Text Solution
|
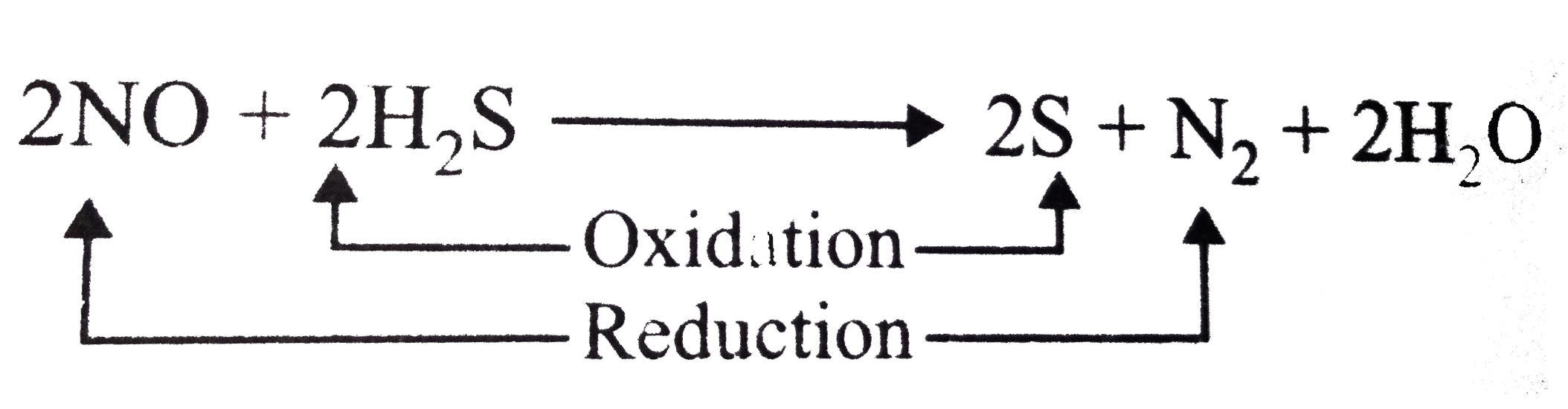
- NO and H(2)S both the pollutants of air. NO is H(2)S remover while H(2...
Text Solution
|
- The region which is greatly affected by air pollution is
Text Solution
|
- Classical smog occurs in places of :
Text Solution
|
- Which of the following is not a herbicide?
Text Solution
|
- Which of the following is not involved in the formation of photochemic...
Text Solution
|
- Which is not the constituent of photochemical smog?
Text Solution
|
- DDT is
Text Solution
|
- Smog can be controlled by
Text Solution
|
- Domestic waste mostly constitute:
Text Solution
|
- The gas leaked from a stronge tank of the Union Carbide plant in Bhopa...
Text Solution
|
- Which is not correct statement for classical smog?
Text Solution
|
- Which of the following is the primary precursor of photochemical smog?
Text Solution
|
- Disinfection of water removes
Text Solution
|
- A common disnfectant used in village wells is
Text Solution
|
- When chlorine is added to water before it enters the distribution syst...
Text Solution
|
- The amount of chlorine avialable in water after disinfection is called
Text Solution
|
- If the organic content in water is high, the type of chlorination to b...
Text Solution
|
- Algae growth in water is controlled by
Text Solution
|