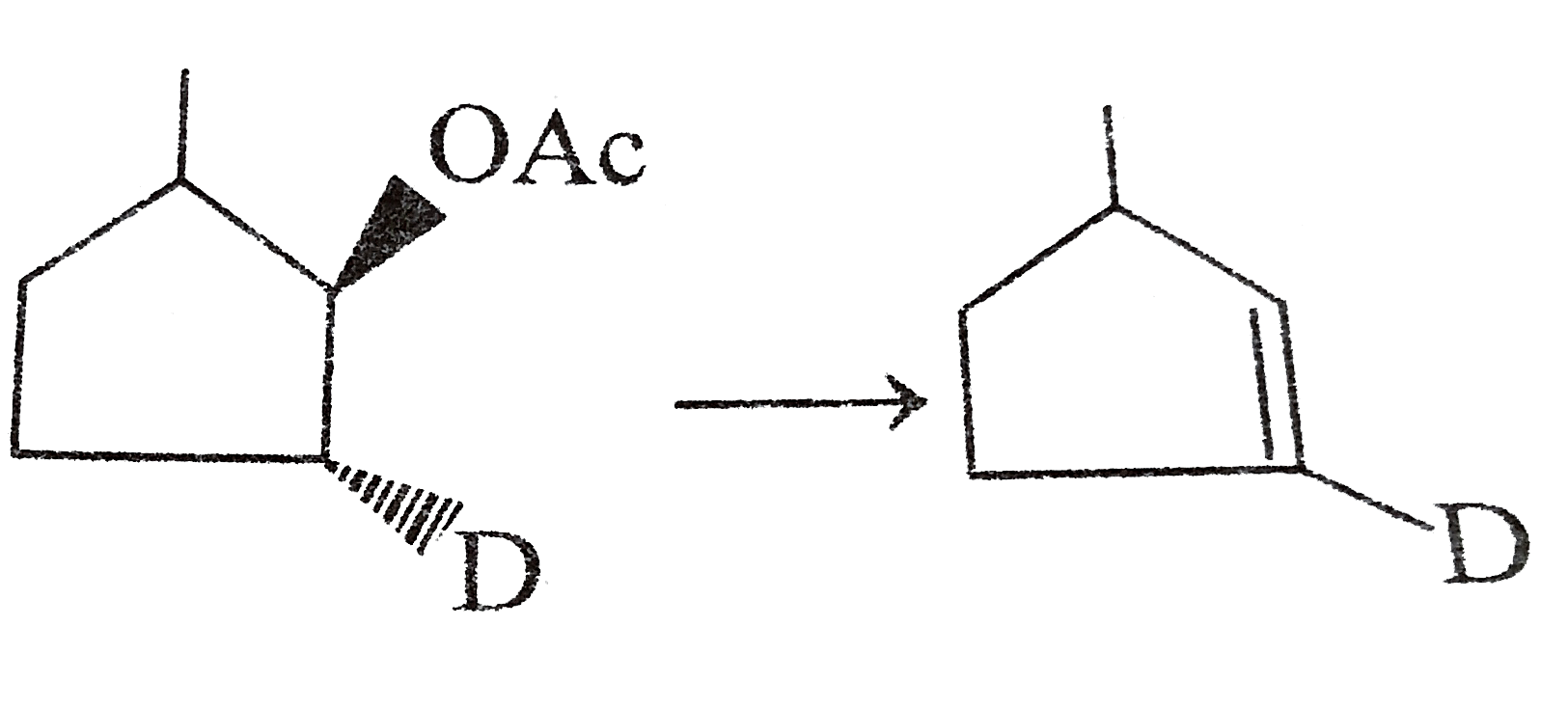Similar Questions
Explore conceptually related problems
Recommended Questions
- Statement-I: Product of pyrolysis of Statement-II: C-H bond is weake...
Text Solution
|
- Statement-I: Product of pyrolysis of Statement-II: C-H bond is weake...
Text Solution
|
- Why do the C-C bonds rather than the C-H bonds break during pyrolysis ...
Text Solution
|
- Statement-1: Oxygen is more electronegative than sulphur, yet H(2)S is...
Text Solution
|
- Explain each statement using resonance theory. (a) The indicated C-...
Text Solution
|
- Statement-1 : Halogens are more reactive than interhalogens. Statement...
Text Solution
|
- Although C-D bond is stronger than C-H bond, yet (CH(3))(3)C^(+) (i) i...
Text Solution
|
- During pyrolysis of alkanesC-C bonds break rather than C-H bonds Why?
Text Solution
|
- Assertion (A): CIF is more reactive than F(2). Reason (R ): The F-F ...
Text Solution
|