Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROSTATICS
PRADEEP|Exercise CURIOSITY QUESTIONS|4 VideosELECTROSTATICS
PRADEEP|Exercise VERY SHORT ANSWER QUESTIONS|5 VideosELECTROSTATICS
PRADEEP|Exercise CONCEPTUAL PROBLEMS|8 VideosELECTRONIC DEVICES
PRADEEP|Exercise Fill in the Blanks|1 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
PRADEEP|Exercise Competition Focus (Multiple Choice Questions)|2 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ELECTROSTATICS-SHORT ANSWER QUESTIONS
- Define an equipotential surface. Draw equipotential surfaces : (i) i...
Text Solution
|
- Establish realation between electric field strength and force.
Text Solution
|
- What is an equipotential surface ? Write three properties Sketch ...
Text Solution
|
- Explain electrostatic shiedling with examples.
Text Solution
|
- Show that the electric field at the surface of a charged conductor is ...
Text Solution
|
- What is a surface density of charge ? Show that surface density of cha...
Text Solution
|
- Consider a coin, It is electrically neutral and contains equal amounts...
Text Solution
|
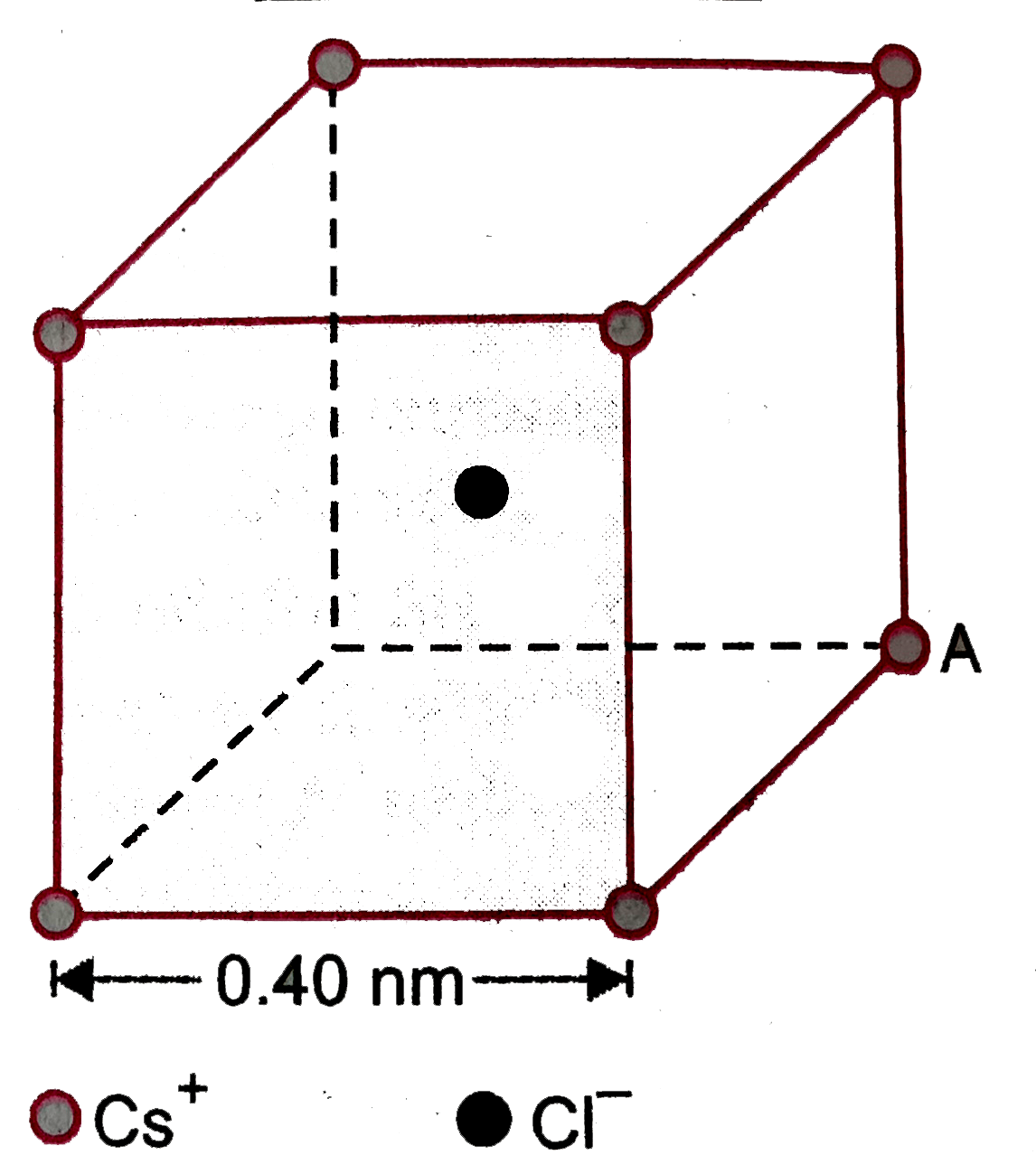
- Figure represents a crystal unit of cesium chloride, CsCl. The cesium ...
Text Solution
|
- Five charges, q each are placed at the corners of a regular pentagon o...
Text Solution
|