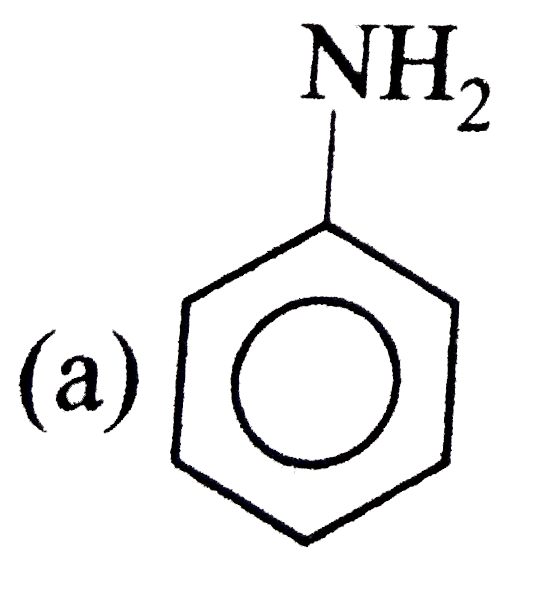
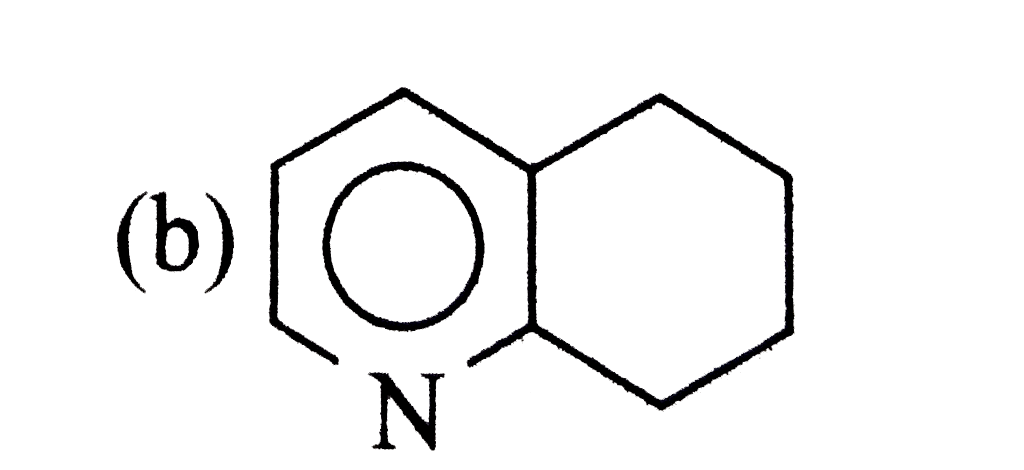
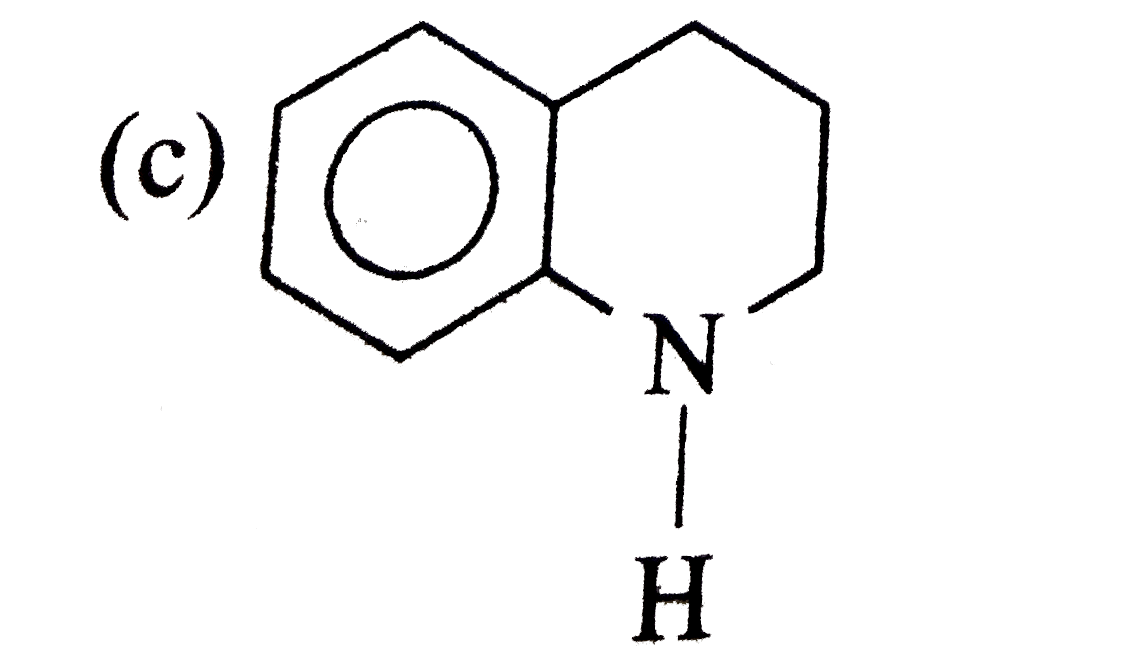
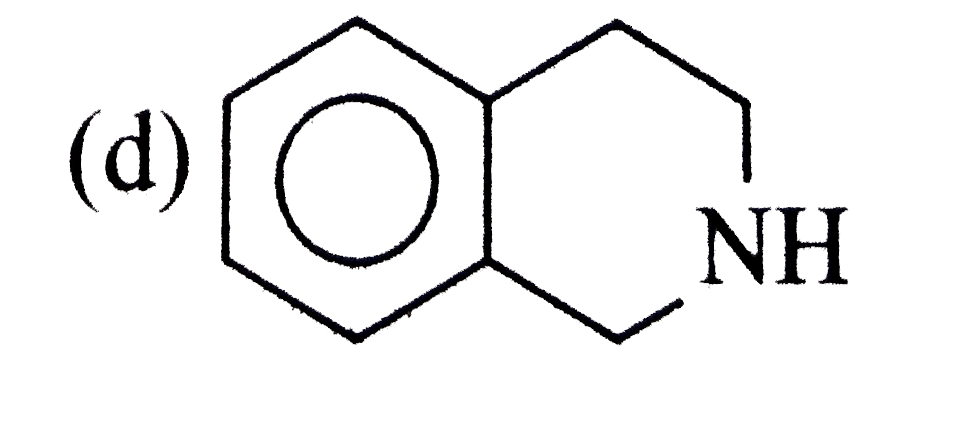
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Bond Fission, Reagents, Reactive Intermediates And Their Stability|31 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Structure Isomerism|28 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|60 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosSTATES OF MATTER
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY-Acid And Basic Strength
- Out of the following compunds, which will have a zero diopole moment?
Text Solution
|
- Arrange the following in increasing order of basic strength
Text Solution
|
- Choose the strongest base among the following:
Text Solution
|
- Which of the following has lowest melting point?
Text Solution
|
- Select the basic strength order of following molecules
Text Solution
|
- What is formed in the above reaction?
Text Solution
|
- Consider thiol anion (RS^(Theta)) and alkoxy anion (RO^(Theta)). Which...
Text Solution
|
- The correct basic strength order of the following anions is:
Text Solution
|
- Which of the following has the most acidic hydrogen ?
Text Solution
|
- Which of the following cannot be a base?
Text Solution
|
- Arrange above phenol in increasing order of pK(a) value:
Text Solution
|
- Arrange the following compounds in decreasing order of their acidic st...
Text Solution
|
- Which compound would be least soluble in water?
Text Solution
|
- Identify the correct order of boiling points of the following compound...
Text Solution
|
- Most acidic hydrogen is present in:
Text Solution
|
- The decreasing order of electron density on the ring is:
Text Solution
|
- The correct order of increasing basicity of the given conjugate bases ...
Text Solution
|
- Among the following compounds, the most acid is:
Text Solution
|
- Correct dipole moment order is: underset((p))(CH(2)=CH-Cl),underset(...
Text Solution
|
- Which of the following compound is the strongest base?
Text Solution
|