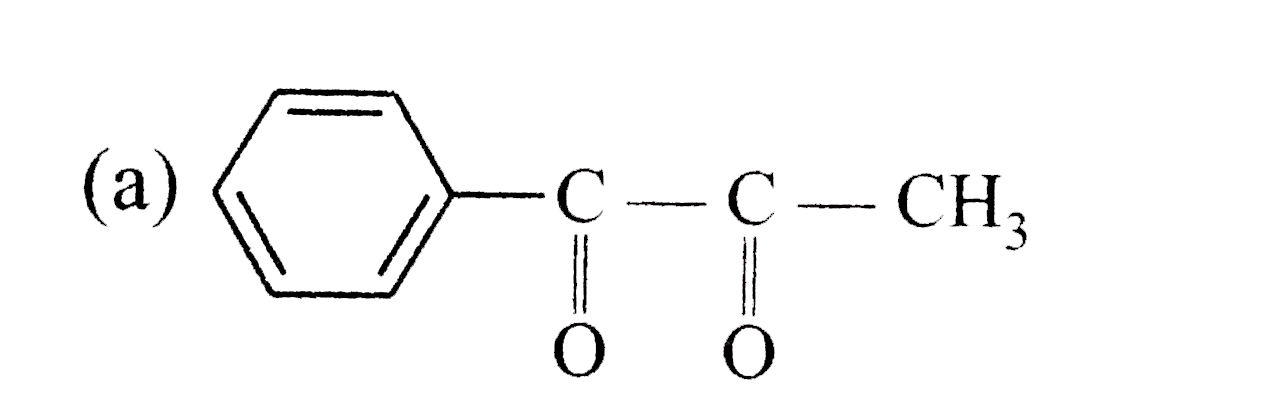
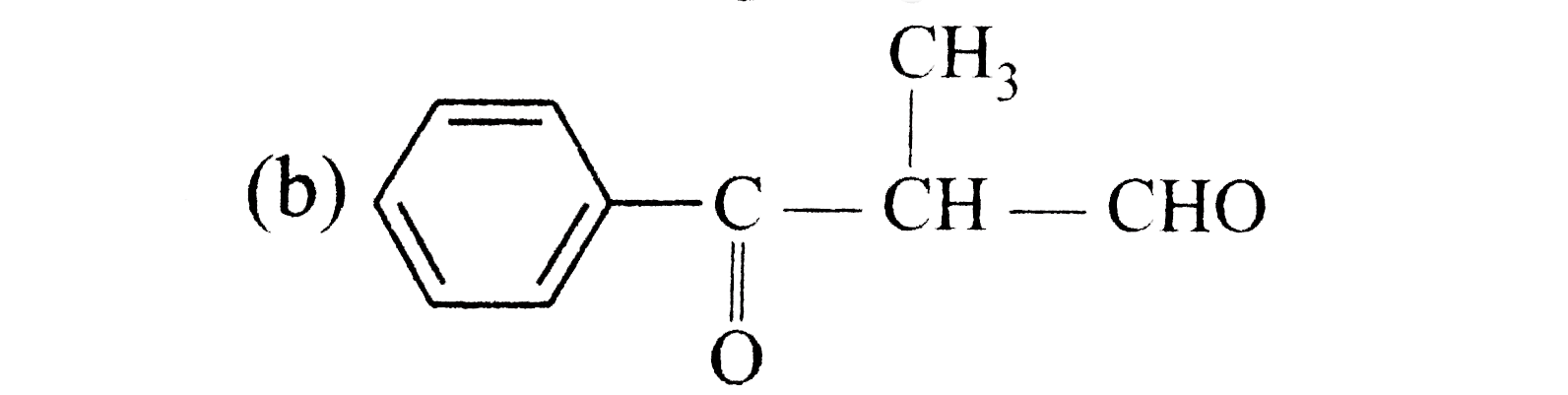
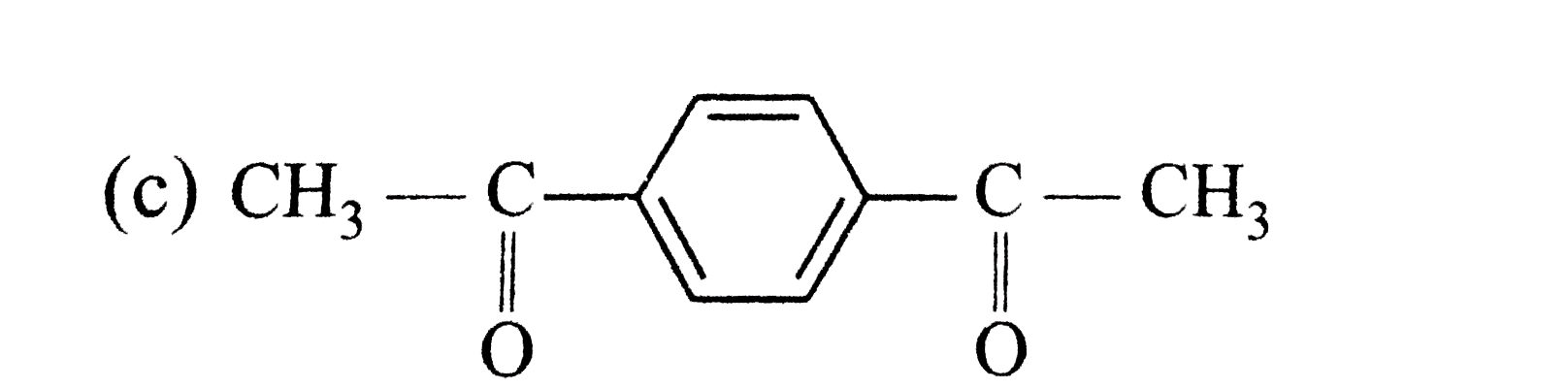
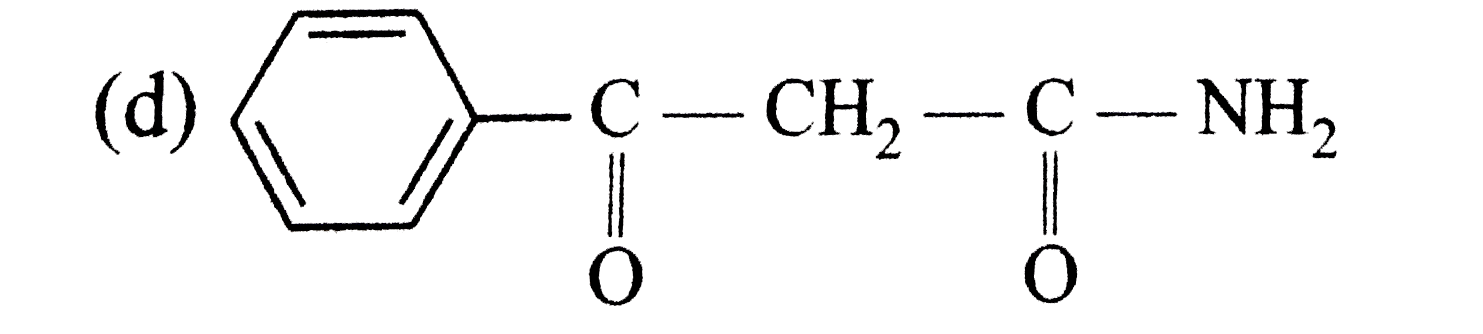A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Geometrical Isomerism|47 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Optical Isomerism|53 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Structure Isomerism|28 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosSTATES OF MATTER
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY-Tautomerism
- Among the following compounds, one that will not shown keto-enol tauto...
Text Solution
|
- Which statements about tautomerism is incorrect?
Text Solution
|
- The enolic form of acetone contains:
Text Solution
|
- Arrange the following in the increasing order of stability of their mo...
Text Solution
|
- Maximum enolisation takes place in
Text Solution
|
- In hexane-2,4-dione, how many different mono-enols are possible?
Text Solution
|
- Tautomerism is not exhibited by
Text Solution
|
- Keto-enol tautomerism is observed in
Text Solution
|
- Tautomerism will be exhibited by
Text Solution
|
- What is the correct order of equilibrium enol content of the following...
Text Solution
|
- Which among the following compounds will give maximum enol content in ...
Text Solution
|
- Which of the following has greater enol content than keto counterpart?
Text Solution
|
- Which of the following has highest % enol content in the liquid?
Text Solution
|
- In which of the following compound, enol form exists?
Text Solution
|
- Arrange the following in the increasing order of acidic strength (I)...
Text Solution
|
- The most stable keto isomer of the following compound is
Text Solution
|