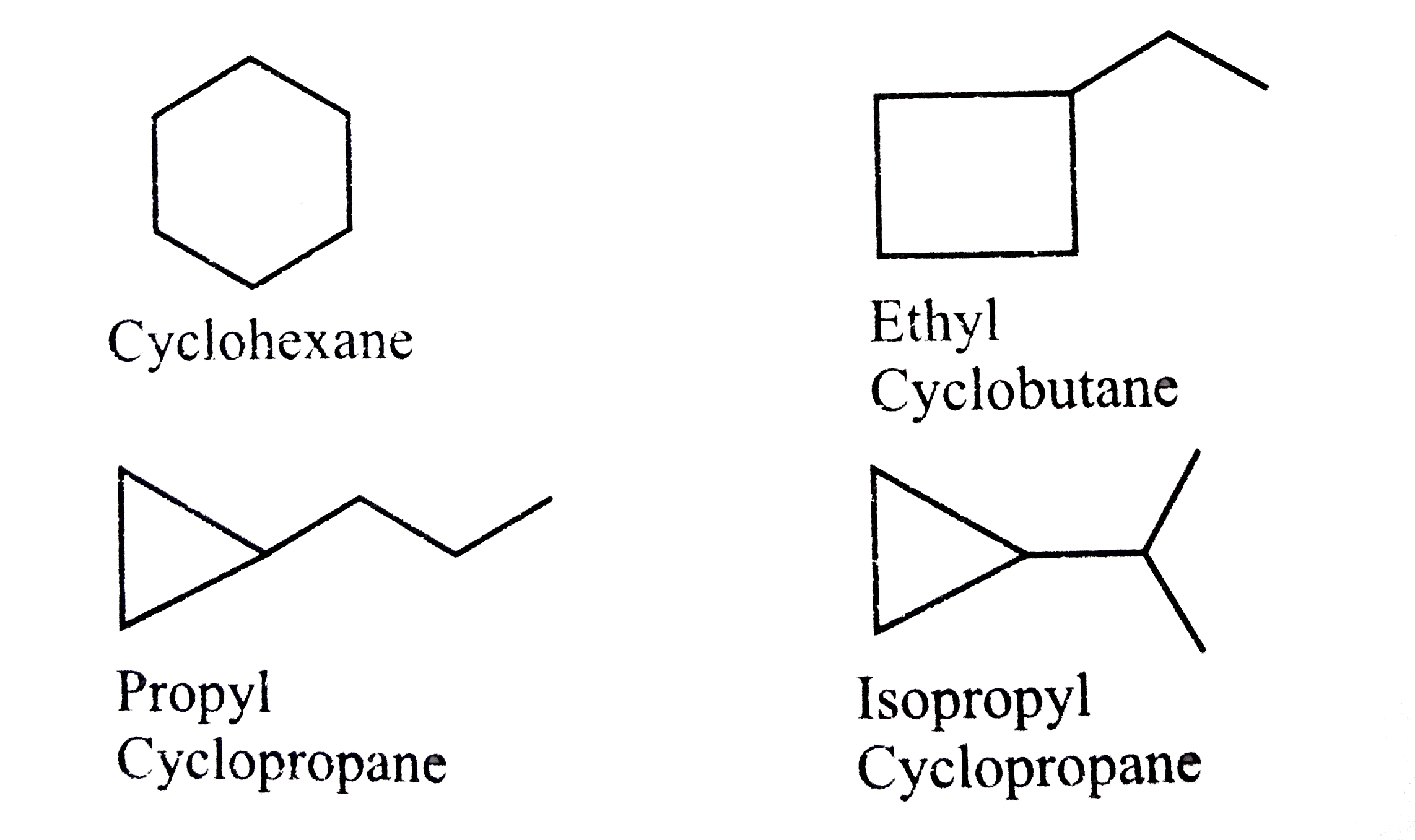
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Nomenclature Of Alkenes And Alkynes|22 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Nomenclature Of Compounds Containing One Functional Group|36 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Section D - Chapter End Test
- A hydrocarbon X has molar mass 84 but contains no double or triple bon...
Text Solution
|
- There is something wrong in the name of the compound named : 2-bromo...
Text Solution
|
- An organic compound contains 49.3% carbon 6.84% hydrogen and its vapou...
Text Solution
|
- Ethyoxyethene is written as follows, H(2)C=CH-O-CH(2)-CH(3). The s...
Text Solution
|
- If 0.228 g of silver salt of dibasic acid gabe a residue of 0.162 g of...
Text Solution
|
- Which of the following compound is unsaturated hydrocarbon.
Text Solution
|
- 0.0833 mol of carbohydrate of empirical formula CH(2)O contain 1 g of...
Text Solution
|
- The correct IUPAC name of the compound is :
Text Solution
|
- 116 mg of a compound on vaporisation in a Victor - Meyer's apparatus d...
Text Solution
|
- According to IUPAC convention, the correct name of the following compo...
Text Solution
|
- A gas mixture contains 50% helium and 50% methane by volume. What is t...
Text Solution
|
- What is the correct IUPAC name of the following compound ?
Text Solution
|
- 0.5g of hydrocarbone gave 0.9 g water on combustion . The percentage ...
Text Solution
|
- What is the correct IUPAC name of the compound shown below?
Text Solution
|
- Lassaigne's test for the detection of nitrogen will fail in case of :
Text Solution
|
- The correct IUPAC name of the compound shown below
Text Solution
|
- Camphor is often used in molecular mass determination because
Text Solution
|
- In Kjeldahl's method, nitrogen present is estimated as :
Text Solution
|
- How many H- atoms are present in 0.046 g of ethanol ?
Text Solution
|
- In the compound CH(2)=CH-CH(2)-CH(2)-C-=CH the C(2)-C(3) bond is of
Text Solution
|
- A hydrocarbon contains 10.5 gm carbon and 1 gm hydrogen. Its 2.4 gm ha...
Text Solution
|