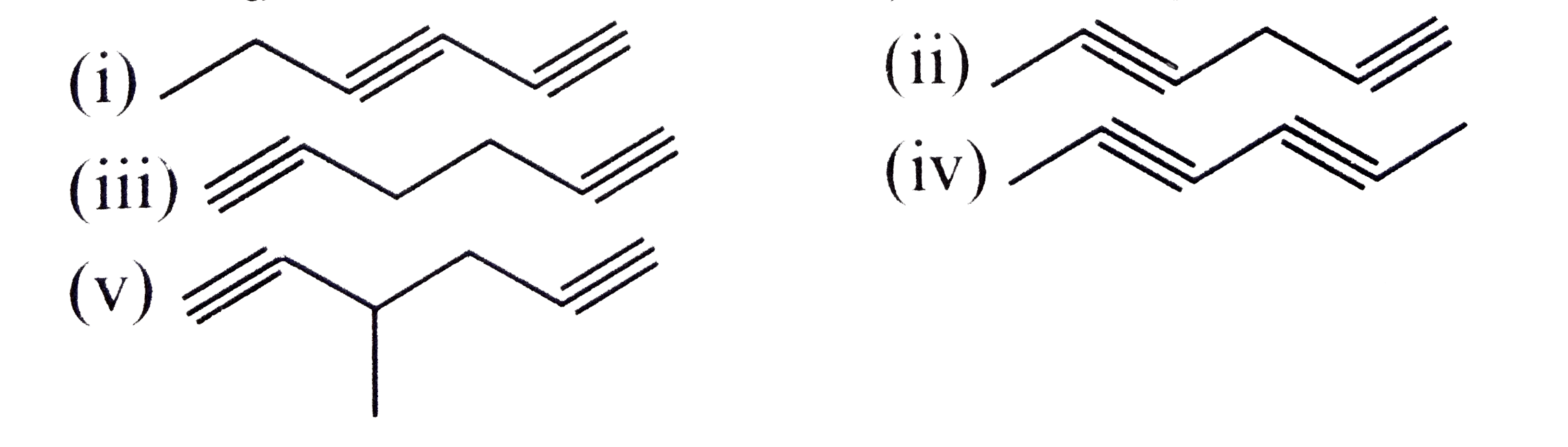A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Nomenclature Of Compounds Containing One Functional Group|36 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Naming Of Polyfunctional Compounds|29 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Nomenclature Of Alkenes And Alkynes
- The IUPAC name of
Text Solution
|
- 3-methyl pent-1,3-diene is
Text Solution
|
- Which among the following is the correct IUPAC name of isoamylene ?
Text Solution
|
- Degree of unsaturation in is
Text Solution
|
- What is the correct IUPAC name of the following compound ?
Text Solution
|
- Select the structure with correct numbering in the chain :
Text Solution
|
- A hydrocarbon has molecular formula X(C(6)H(6)). Also, X has triple bo...
Text Solution
|
- Assuming a hydrocarbon C(5)H(10) without any double bond, how many dif...
Text Solution
|
- The correct IUPAC name of the compound
Text Solution
|
- What is the IUPACname of the following compound ?
Text Solution
|
- The correct structure of 2-Ethyl -3-methyl hex-1,4-diene
Text Solution
|
- What is the correct IUPAC name of the compound shown below ?
Text Solution
|
- The correct IUPAC name of 2-ethyl-3-pentyne is
Text Solution
|
- Which is the correct structure of 5,6,6- trimethyl 3- heptyne ?
Text Solution
|
- What is the correct IUPAC name of the compound shown below ?
Text Solution
|
- What is correct IUPAC name of the following compound ?
Text Solution
|
- The molecular formula of the first member of the family of alkenynes a...
Text Solution
|
- In the compound CH(2)=CH-CH(2)-CH(2)-C-=CH the C(2)-C(3) bond is of
Text Solution
|
- There is something wrong in the name of the compound named : 2-bromo...
Text Solution
|
- What is the correct IUPAC name of the following compound ?
Text Solution
|