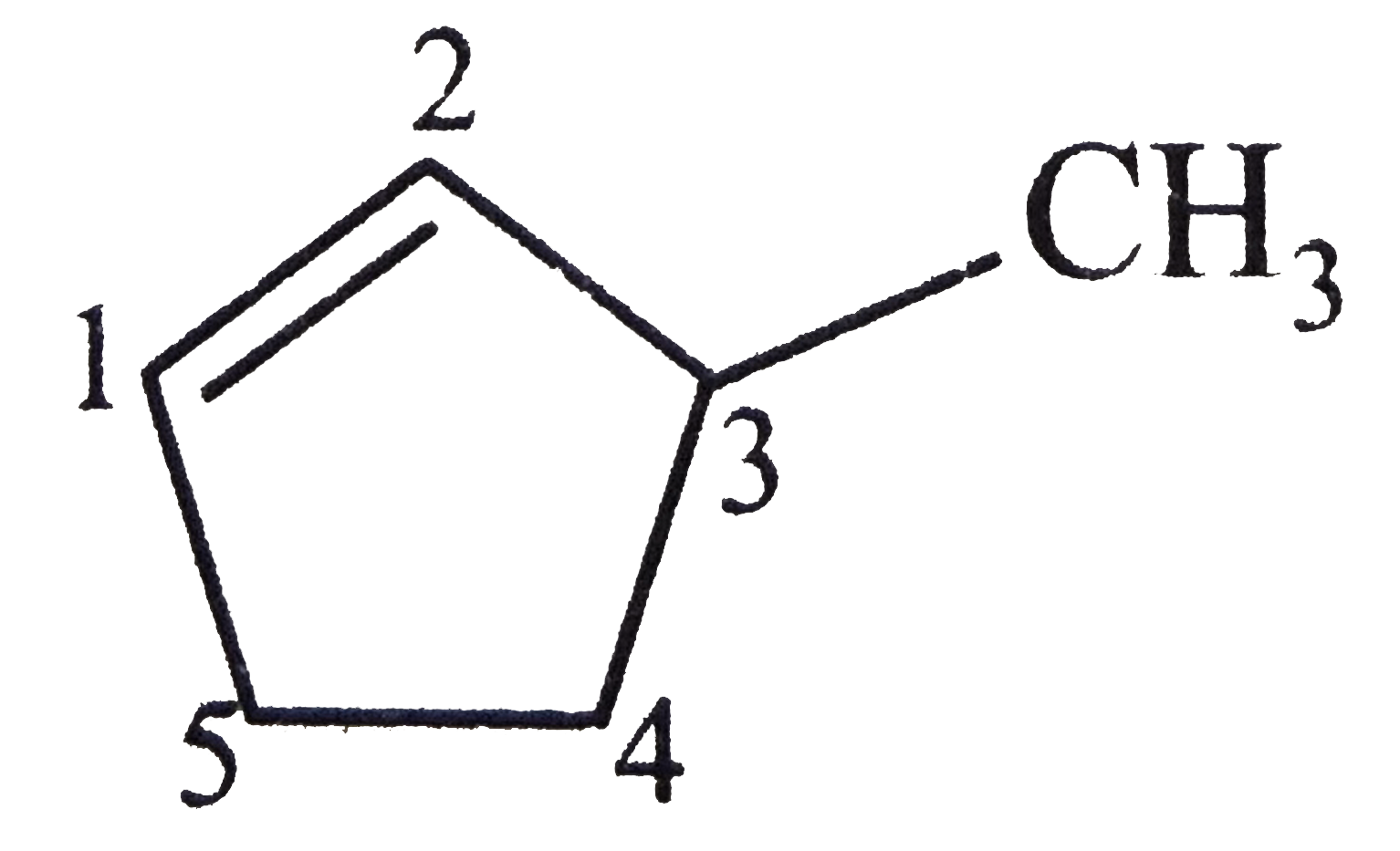
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise AIPMT/NEET/AIIMS Problem|34 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Assertion-Reasoning Questions|5 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Purification Of Organic Compounds|52 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Section B - Assertion Reasoning
- Assertion : Glycerol is purified by distilled under reduced pressure. ...
Text Solution
|
- Assertion : The locant (2,6,7) is preferred over the locant (3,4,8). ...
Text Solution
|
- Assertion : The yield in organic reactinos are 100% Reason : The or...
Text Solution
|
- Assertion : The IUPAC name of CH(3)CH=CH-C CH is pent-2-ene-4-yne and ...
Text Solution
|
- Assertion : Solid - Liquid mixtures are separated by crystallization. ...
Text Solution
|
- Assertion : The correct IUPAC name for the compound is (1-methylethyl...
Text Solution
|
- Assertion : is called cyclohexane nitrile. Reason: It contains six...
Text Solution
|
- Assertion : Sodium extract is prepared for identification of elements ...
Text Solution
|
- Assertion : The IUPAC name for the compound Reason : Ethyl(C(2)H(...
Text Solution
|
- Assertion (A) : The IUPAC name of the citric acid is 2-hydroxy-propane...
Text Solution
|
- Assertion : Moving phase is liquid and stationary phase is solid in pa...
Text Solution
|
- Assertion : Thiophene present in commercial benzene as an impurity can...
Text Solution
|
- Assertion (A) : Pentane and 2-methy1 pentane are homolo-gues. Reason...
Text Solution
|
- Assertion : Refining of petroleum involves fractional distillation. ...
Text Solution
|
- Assertion : Butane and 2-methyl butane are chain isomers. Reason : B...
Text Solution
|
- Assertion : Neopentane is chain isomer of n-pentane. Reason : Molecu...
Text Solution
|
- Assertion : is 3-methyl cyclopentene. Reason : In numbering, double...
Text Solution
|
- Assertion , Alkanes containing more than three carbone atoms exhibit c...
Text Solution
|
- Assertion (A) : All the C atoms of but-2-ene lie in one plane Reason...
Text Solution
|
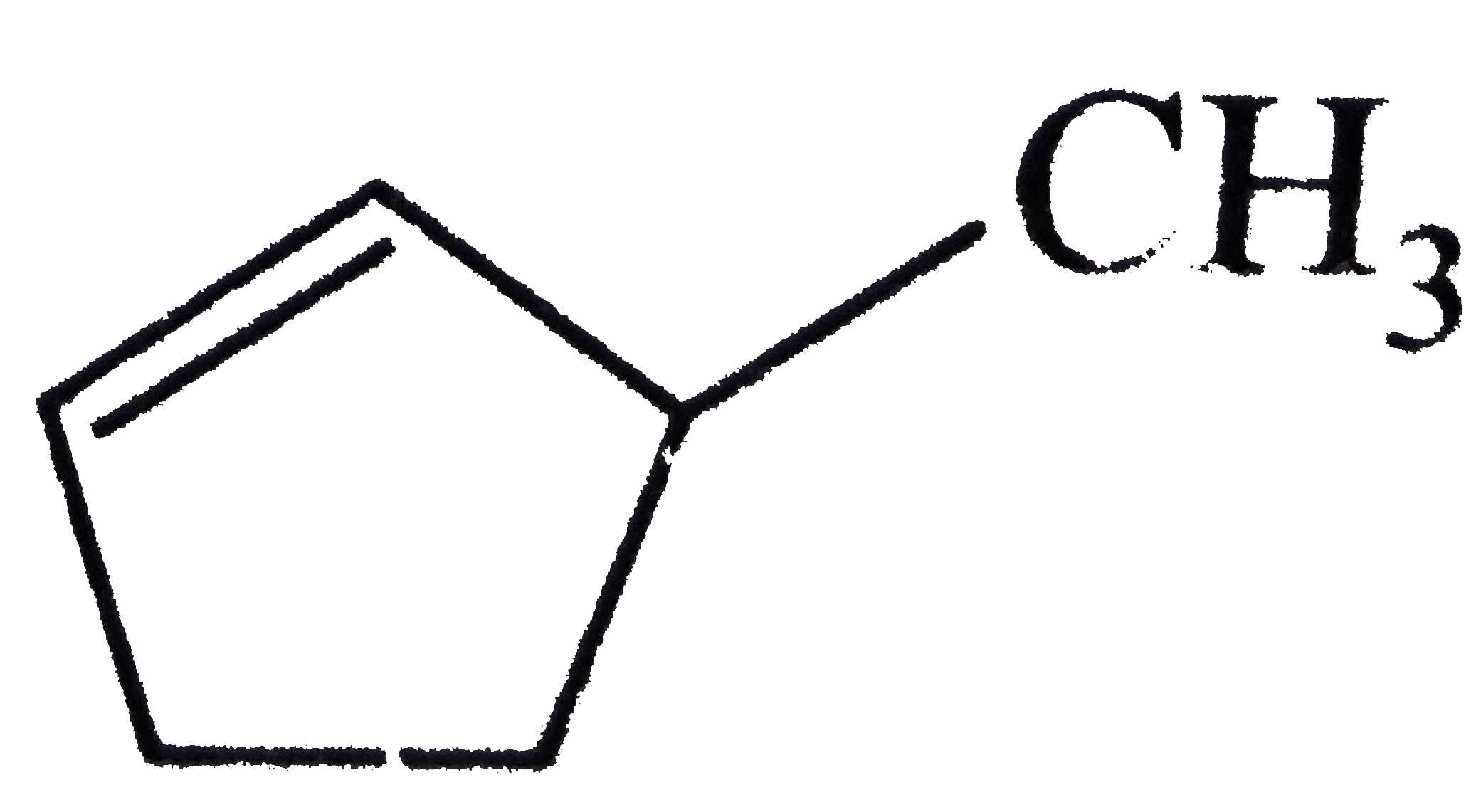 is `3-`methyl cyclopentene.
is `3-`methyl cyclopentene.