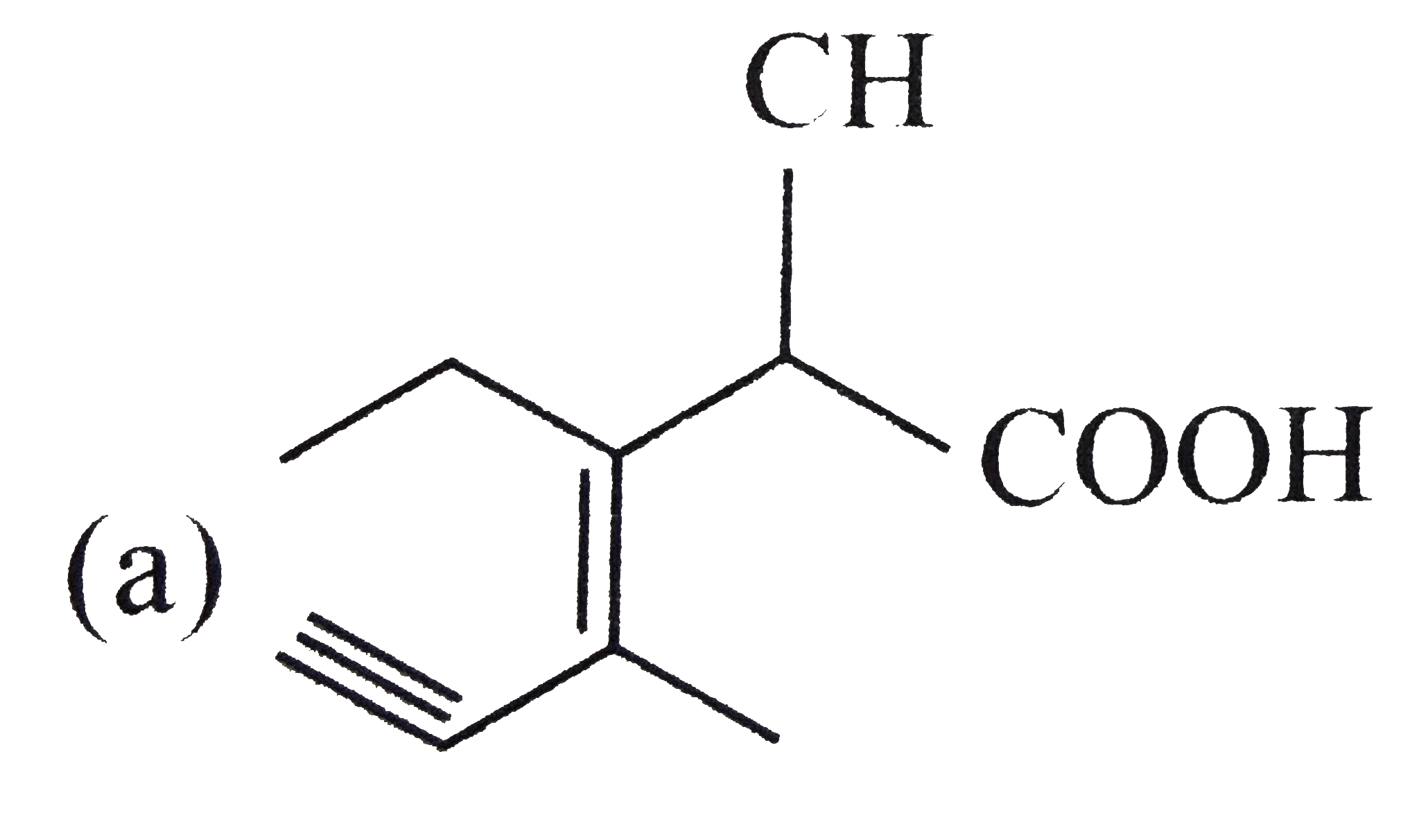
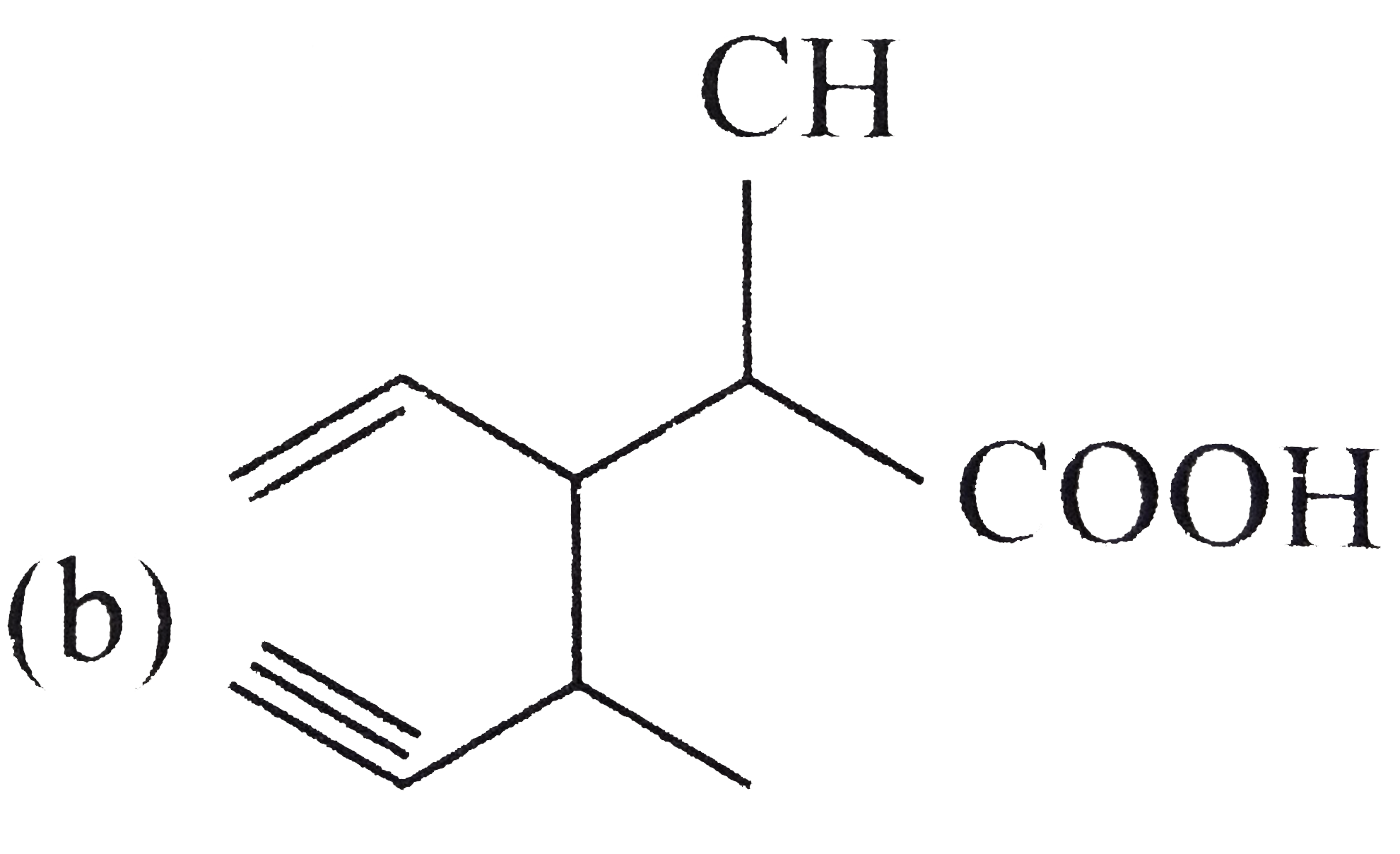
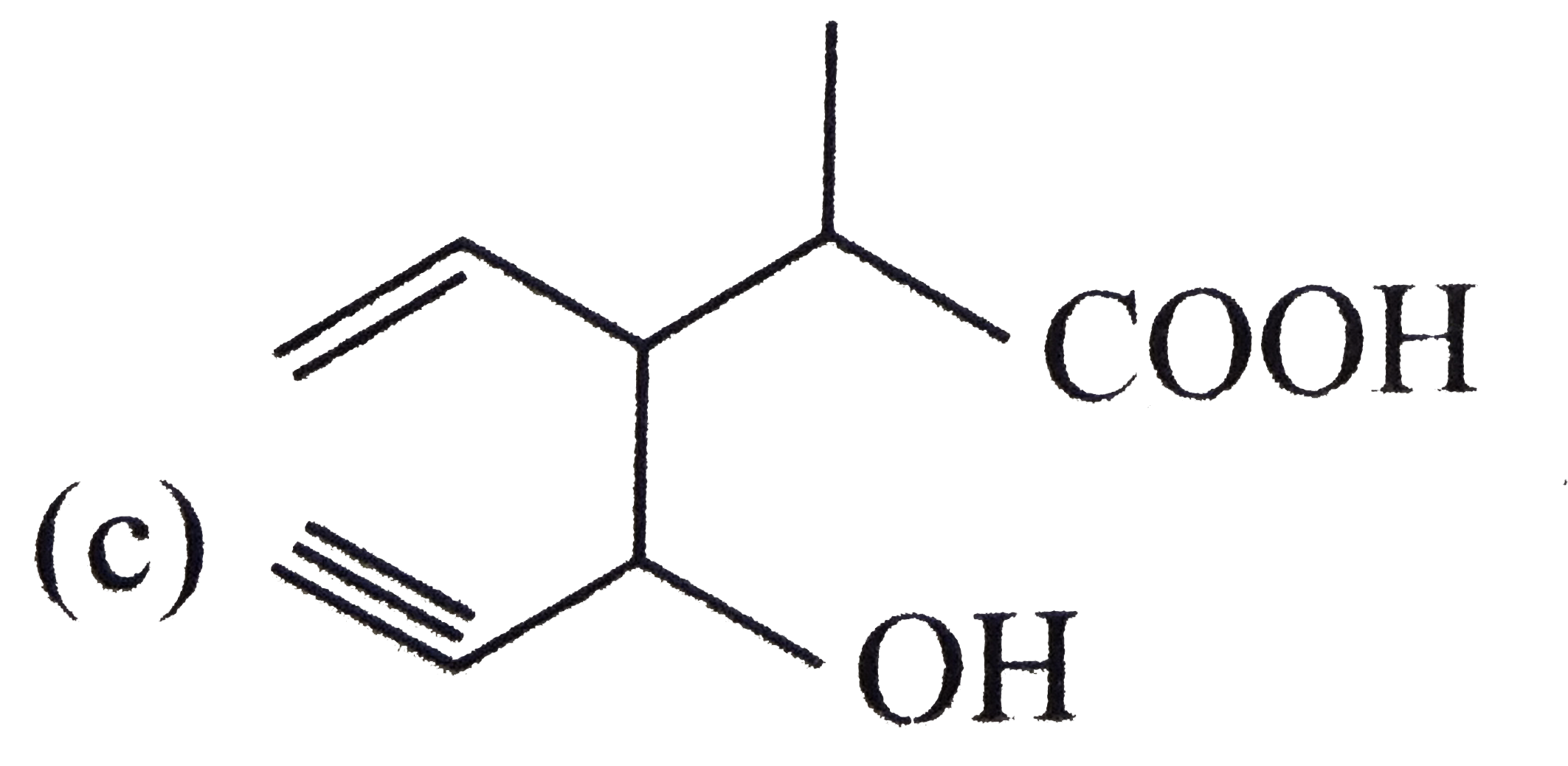
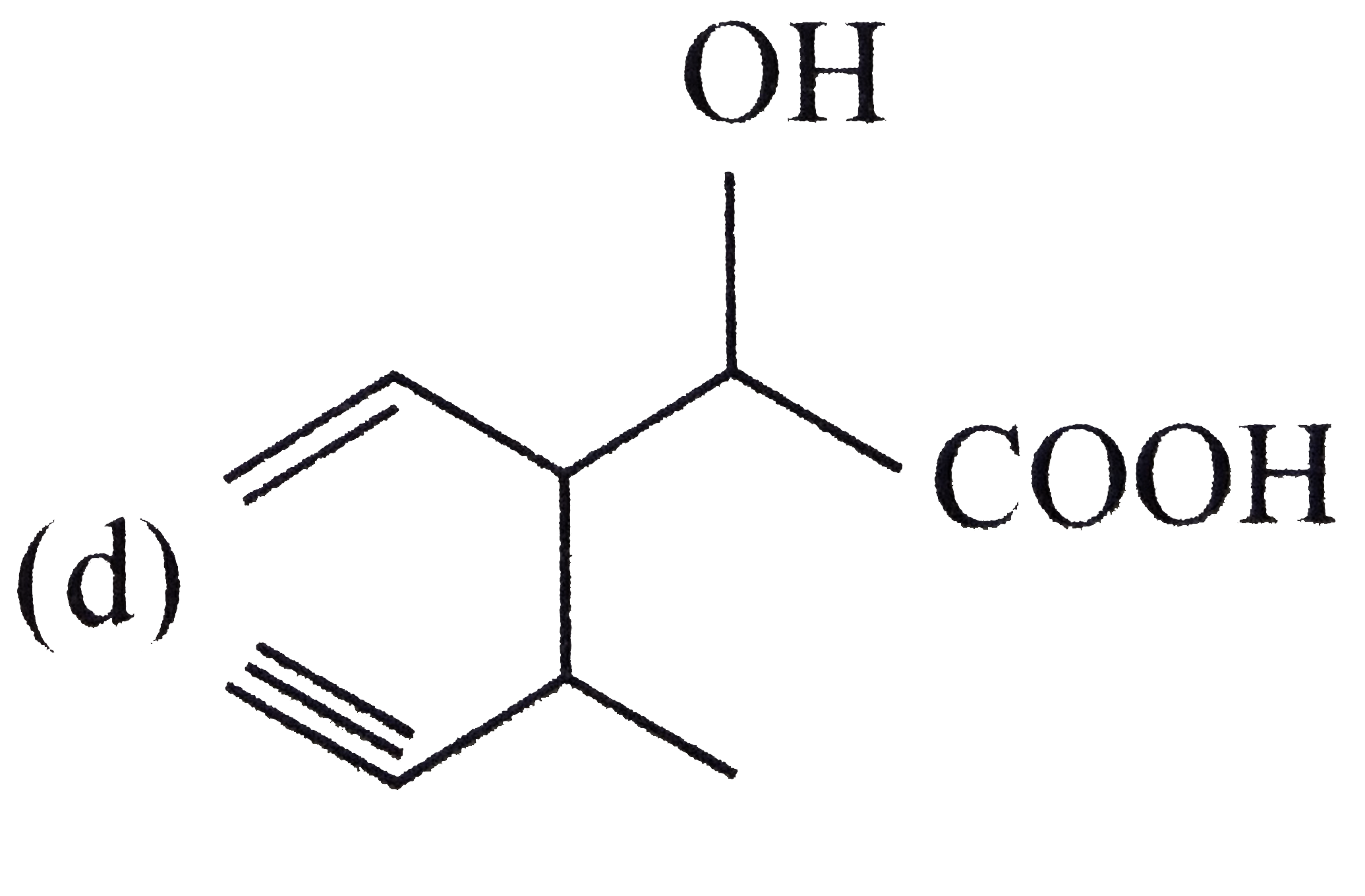
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Assertion-Reasoning Questions|5 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
A2Z|Exercise Section B - Assertion Reasoning|19 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CLASSIFICTION, PURIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-AIPMT/NEET/AIIMS Problem
- The LaSSaigen's extract is boiled with conc. HNO(3) while testing for...
Text Solution
|
- The correct IUPAC name of the compound, is
Text Solution
|
- Which nomenclature is not according to IUPAC system?
Text Solution
|
- Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-meth...
Text Solution
|
- The structure of isobutyl group in an organic compound is
Text Solution
|
- In Duma's method for estimation of nitrogen. 0.25 g of an organic comp...
Text Solution
|
- The most suitable method of separation of a mixture of ortho and para ...
Text Solution
|
- The IUPAC name of the compound is :
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- What hybrid orbitals will form the following compound H(3)C-CH=CH-CH(2...
Text Solution
|
- Empirical formula of a compound is CH(2)O and its vapour density is 30...
Text Solution
|
- An organic compound containing C,H and N have the percentage 40,13.33 ...
Text Solution
|
- If a compound on analysis was found to contain C=18.5%, h=1.55%,Cl=55....
Text Solution
|
- 0.2595 g of an organic substance in a quantitative analysis yielded 0....
Text Solution
|
- In Kjeldahl's method of estimation of N,CuSO(4) acts as
Text Solution
|
- How will you separate a solution ( miscible ) of benzene +CHCl(3) ?
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Write the IUPAC name of CH(3)CH(2)COOH
Text Solution
|
- If 0.228 g of silver salt of dibasic acid gabe a residue of 0.162 g of...
Text Solution
|
- What is IUPAC name of the following ?
Text Solution
|