Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS AND MOLECULAR PHYSICS
IE IRODOV, LA SENA & SS KROTOV|Exercise Transport Phenomena|38 VideosTHERMODYNAMICS AND MOLECULAR PHYSICS
IE IRODOV, LA SENA & SS KROTOV|Exercise Liquids Capillary Effects|25 VideosPHYSICAL FUNDAMENTALS OF MECHANICS
IE IRODOV, LA SENA & SS KROTOV|Exercise Relativistic Mechanics|49 Videos
Similar Questions
Explore conceptually related problems
IE IRODOV, LA SENA & SS KROTOV-THERMODYNAMICS AND MOLECULAR PHYSICS-Phase Transformations
- Write the Van der Waals equation via the reduced parameters pi, v and ...
Text Solution
|
- Knowing the Van der Waals constant, find : (a) the maximum volume wh...
Text Solution
|
- Calculate the temperature and density of carbon dioxide in critical st...
Text Solution
|
- What fraction of the volume of a vessel must liquid ether occupy at ro...
Text Solution
|
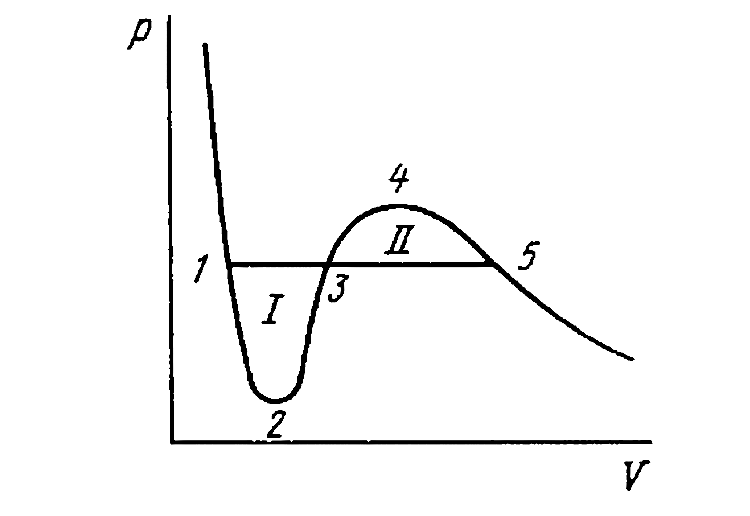
- Demonstrate that the straight line 1-5 corresponding to the isothermal...
Text Solution
|
- What fraction of water supercooled down to the temperature t = -20 ^@C...
Text Solution
|
- Find the increment of the ice melting temperature in the vicinity of 0...
Text Solution
|
- Find the specific volume of saturated water vapour under standard pres...
Text Solution
|
- Assuming the saturated water vapour to be ideal, find its pressure at ...
Text Solution
|
- A small amount of water and its saturated vapour are enclosed in a ves...
Text Solution
|
- Find the pressure of saturated vapour as a function of temperature p(t...
Text Solution
|
- An ice which was initially under standard conditions was compressedup ...
Text Solution
|
- In the vicinity of the triple point the saturated vapour pressure p of...
Text Solution
|
- Water of mass m = 1.00 kg is heated from the temperature t1 = 10^@C up...
Text Solution
|
- The ice with the initial temperature t1 = 0 ^@C was first melted, then...
Text Solution
|
- A piece of copper of mass m = 90 g at a temperature t1 = 90 ^@C was pl...
Text Solution
|
- A chunk of ice of mass m1 = 100 g at a temperature t1 = 100 g was at a...
Text Solution
|
- Molten lead of mass m = 5.0 g at a temperature t2 = 327^@C (the meltin...
Text Solution
|
- A water vapour filling the space under the piston of a cylinder is com...
Text Solution
|
- One mole of water being in equilibrium with a negligible amount of its...
Text Solution
|
 .
. .
.