Similar Questions
Explore conceptually related problems
Recommended Questions
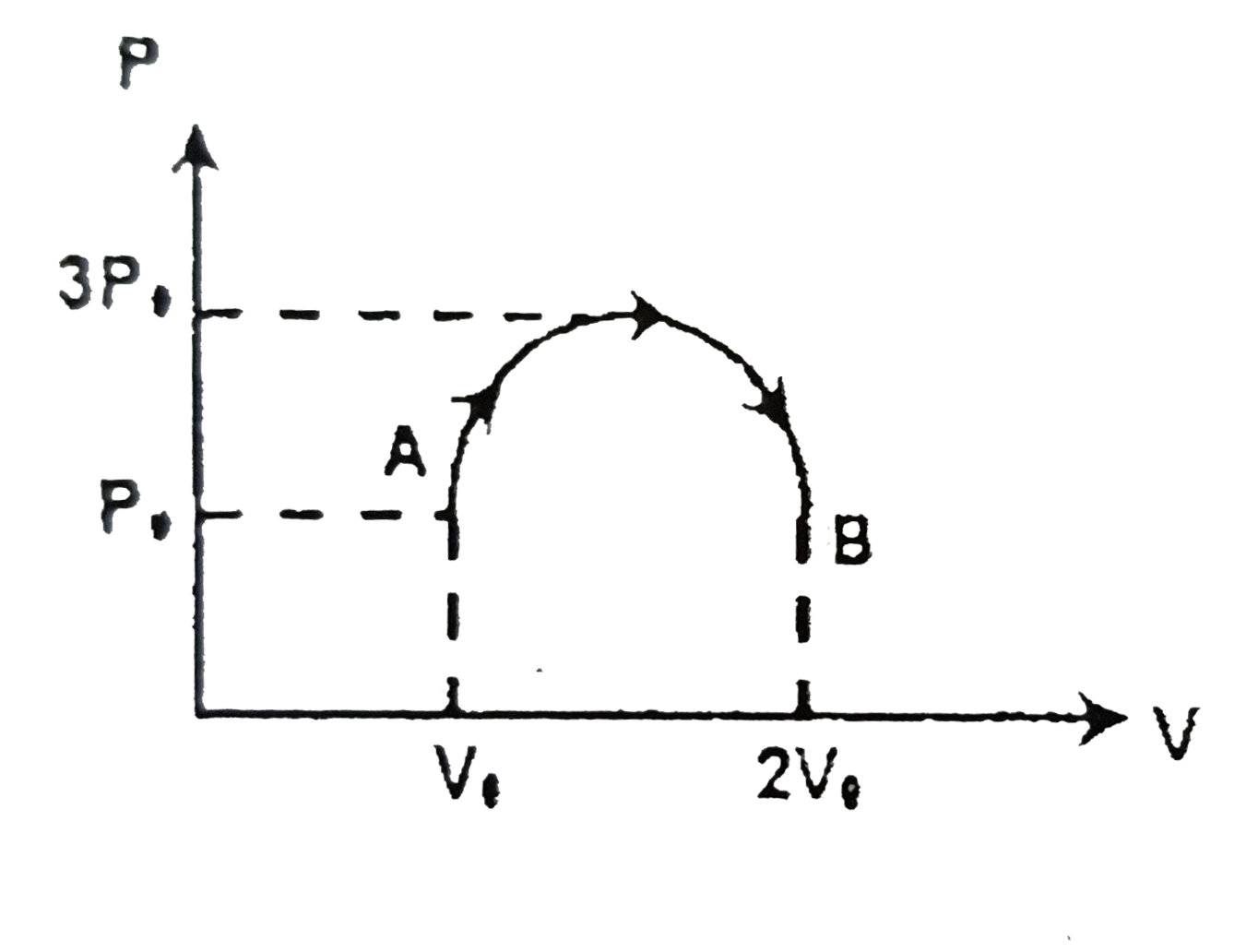
- One mole of an ideal monoatomic gas is taken through the thermodynamic...
Text Solution
|
- One mole of an ideal Po monoatomic gas is taken 4 op through a thermo ...
Text Solution
|
- An ideal monoatomic gas is taken round the cycle ABCDA as shown in the...
Text Solution
|
- One mole of a monoatomic ideal gas undergoes the process ArarrB in the...
Text Solution
|
- V-T graph of a process of monoatomic ideal gas is shown in figure. Hea...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through the thermodynamic...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through a cyclic process ...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- One mole of a monoatomic ideal gas undergoes process AB in given P – V...
Text Solution
|