Similar Questions
Explore conceptually related problems
Recommended Questions
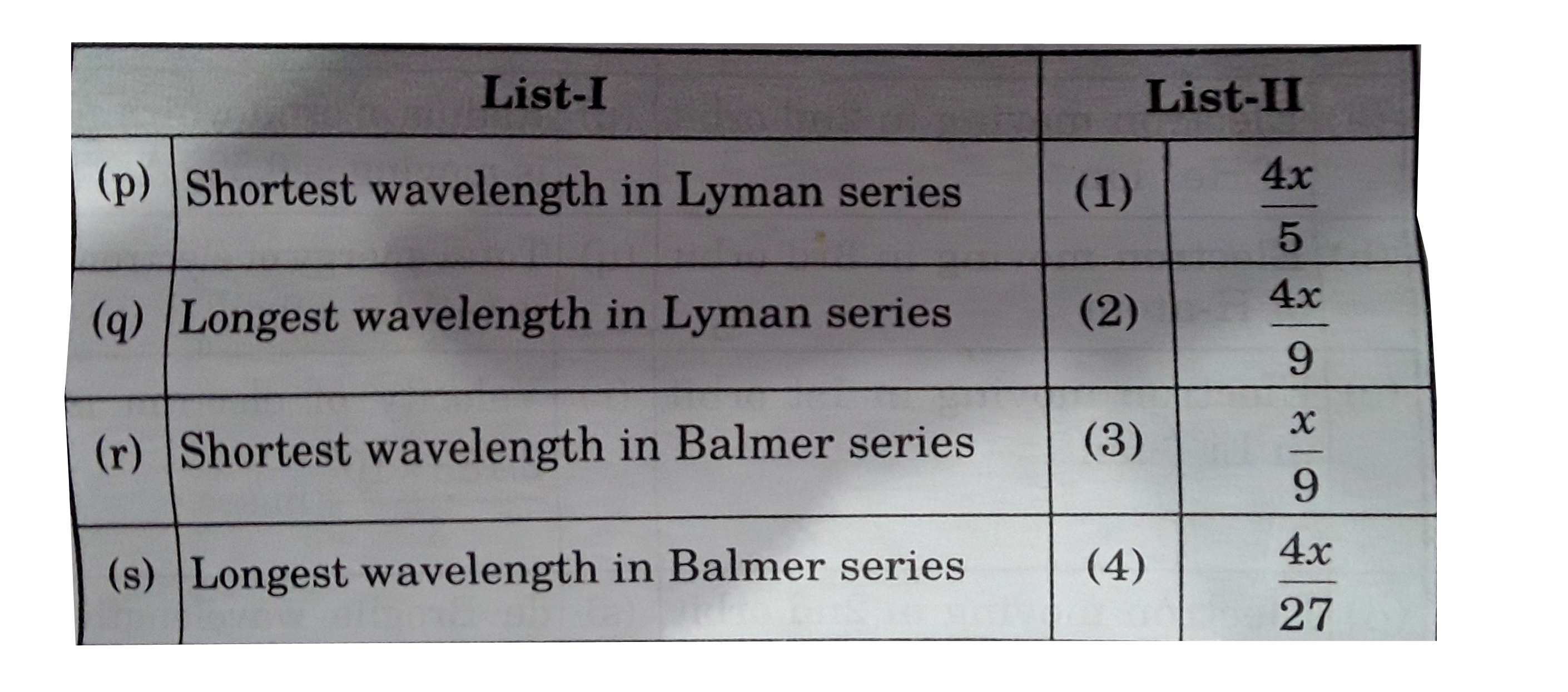
- If the shortest wavelength of spectral line of H-atom in Lyman series ...
Text Solution
|
- If the shortest wavelength of spectral line of H-atom in Lyman series ...
Text Solution
|
- In a scale of 10^(-18)m, match the particle with respect to their prob...
Text Solution
|
- Match the following : Codes : {:((a),q,r,p,s),((b),r,q,p,s),((c ),q,r,...
Text Solution
|
- Match the following lists (where [x] represents the greatest integer f...
Text Solution
|
- Consider int(x^(3)+3x^(2)+2x+1)/(sqrt(x^(2)+x+1))dx =(ax^(2)+bx+c)...
Text Solution
|
- एक समांतर श्रेढ़ी में , S (p ) = q ,s (q ) =P तथा इसके प्रथम...
Text Solution
|
- The shortest wavelength of spectral line in Lyman series is 912 Å. The...
Text Solution
|
- if P,q, r, s in R such that p r= 2 ( q+ s) then
Text Solution
|