Similar Questions
Explore conceptually related problems
Recommended Questions
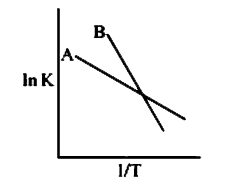
- Plot ln K vs. 1/T for two reactions A and B are given below. Which rea...
Text Solution
|
- Select the incorrect statements about the plots of ln K vs 1/T
Text Solution
|
- The rate constant 'k'. For a reaction varies with temperature 'T' acco...
Text Solution
|
- Plot ln K vs. 1/T for two reactions A and B are given below. Which rea...
Text Solution
|
- Which of the plots of ln K vs (1/T) is/are correct?
Text Solution
|
- The rate constant 'k'. For a reaction varies with temperature 'T' acco...
Text Solution
|
- For a first order reaction, the plot of log[A](t)vs t is linear with a
Text Solution
|
- For the reaction aA+bBrarrcC+dD , the plot of log k vs 1/T is given be...
Text Solution
|
- For the Arrhenius equation, the slope for the plot ln k vs 1/T is
Text Solution
|