Similar Questions
Explore conceptually related problems
Recommended Questions
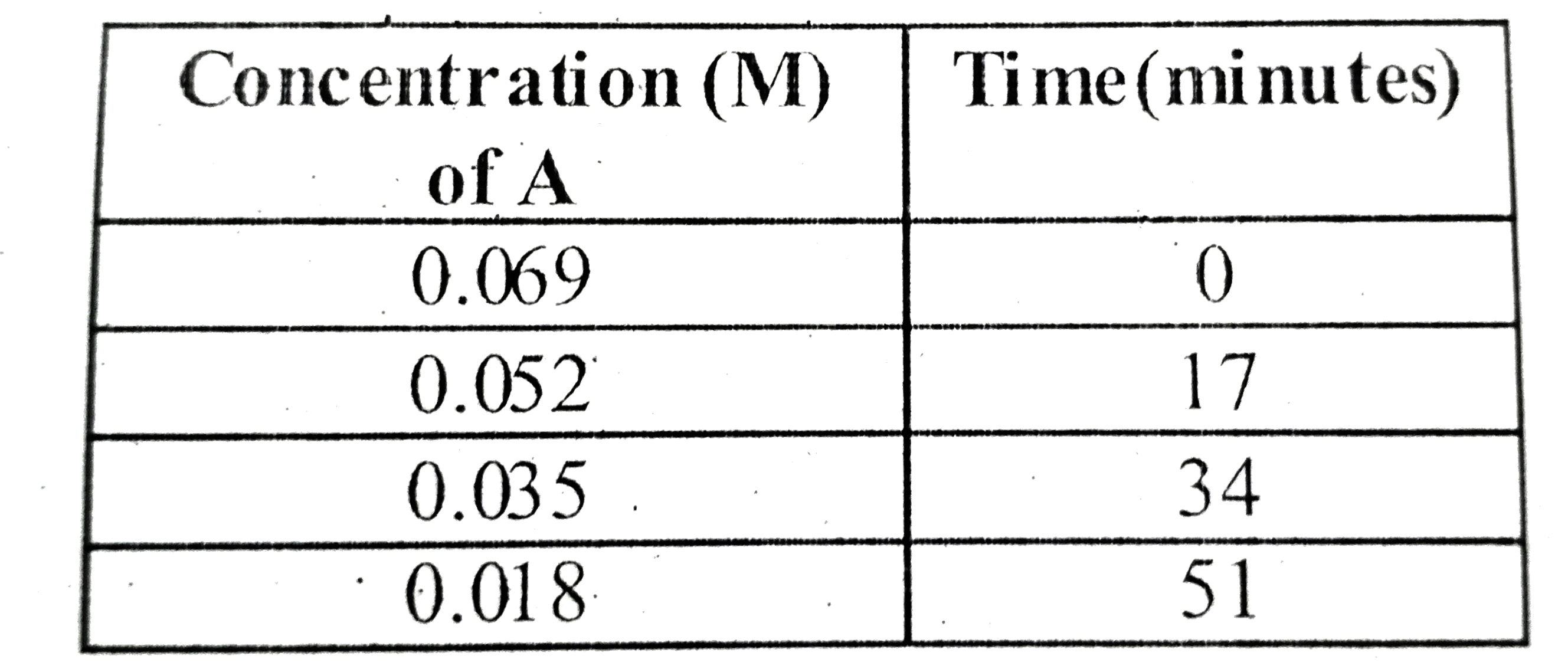
- The concentrations of the reactant A in the reaction A toB at differen...
Text Solution
|
- अभिक्रिया A to B में ,अभिक्रिया की दर, अभिकारकों का सान्द्रण चार ग...
Text Solution
|
- In a reaction A to B the rate of reaction increases two times on inc...
Text Solution
|
- The concentrations of the reactant A in the reaction A to B at differe...
Text Solution
|
- In a reaction to B, the rate of reaction increases two times on increa...
Text Solution
|
- For the reaction 2A+BrarrC, the values of initial rate at different r...
Text Solution
|
- In a reaction A to B, the rate of reaction increases eight times on in...
Text Solution
|
- अभिक्रिया 2A +B to C के लिए अभिकारकों की विभिन्न सांद्रताओं पर प्रारम...
Text Solution
|
- The concentration of the reactant A in the reaction AtoB at different ...
Text Solution
|