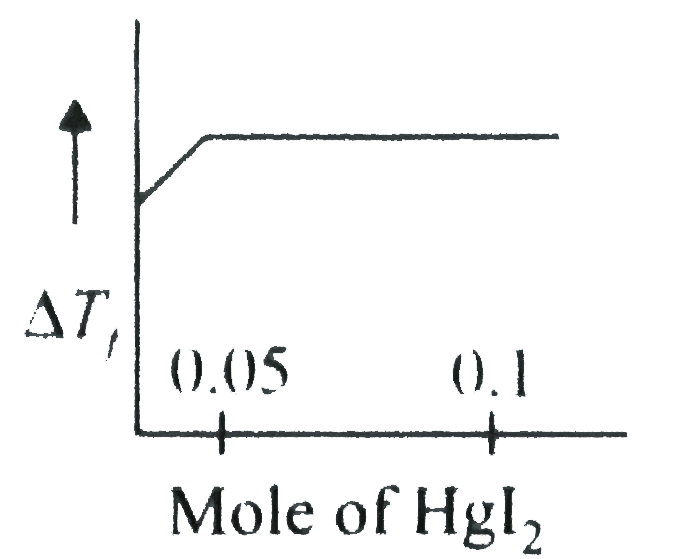
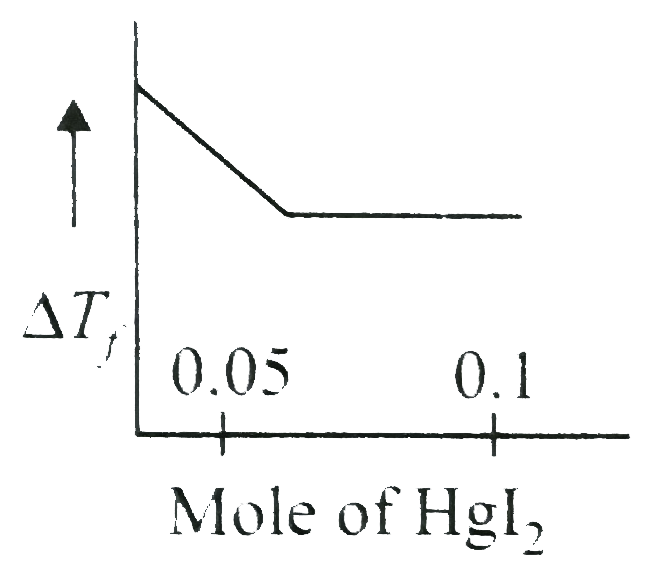
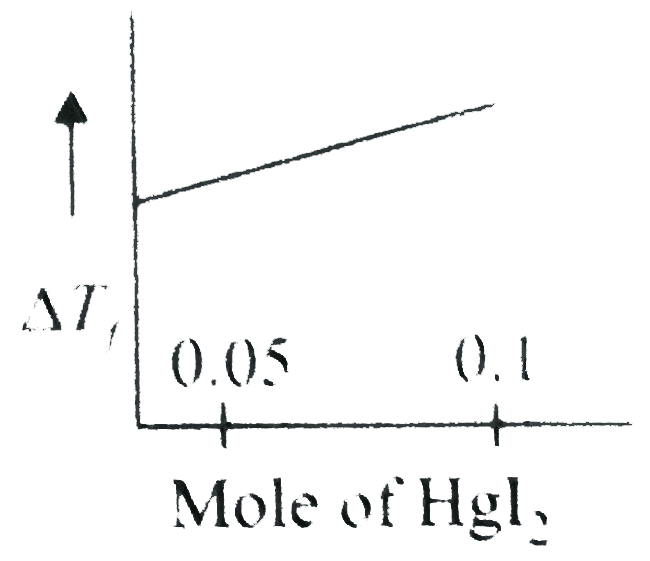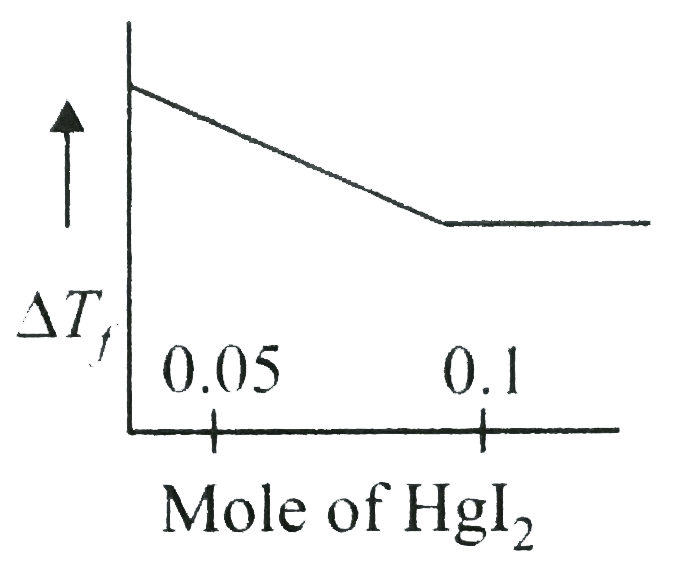A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-SOLUTIONS-Depression Of Freezing Point
- Freezing point of an aqueous solution is -0.186^(@)C. Elevation of boi...
Text Solution
|
- Equimolar solutions of two non-electroytes in the same solvent have:
Text Solution
|
- What should be the freezing point of aqueous solution containing 17g o...
Text Solution
|
- Ethylene glycol is used as an antifreeze in a cold cliamate Mass of et...
Text Solution
|
- 0.48 g of a substance is dissolved in 10.6 g of C(6)H(6).The freezing...
Text Solution
|
- The freezing point of equimolal soution will be highest for :
Text Solution
|
- The freezing point of 0.2molal K(2)SO(4)is -1.1^(@)C. Calculate van't ...
Text Solution
|
- A certain substance A tetramerizes in water to the extent of 80%. A so...
Text Solution
|
- The freezing point of a solution containing 50 cm^(3) of ethylene glyc...
Text Solution
|
- Cryoscopic constant of a liquid
Text Solution
|
- Addition of 0.643g of compound to 50ml of benzene (density =0.879mol^(...
Text Solution
|
- Which of the following solution will have highest freezing point?
Text Solution
|
- 0.48of a substance is dissolved in 10.6g of C(6)H(6).The freezing poin...
Text Solution
|
- What is the freezing point of a solution containing 8.1g Br in 100g wa...
Text Solution
|
- The freezing point of one molal NaClsolution assuming NaClto be 100%di...
Text Solution
|
- Which of the following aqueous molal solution have highest freezing po...
Text Solution
|
- When a solution w g of urea in 1Kg of water is cooled to -0.372^(@)C,2...
Text Solution
|
- NaCl is added to1 litre water to such an extent that DeltaT(f)//K(f) b...
Text Solution
|
- Increasing amoutn of HgI(2)is added to 1 litre of an aqueous solution ...
Text Solution
|
- 3.24gHg(NO(3))(2)(molecules mass324)dissolved in 1000 g of water const...
Text Solution
|