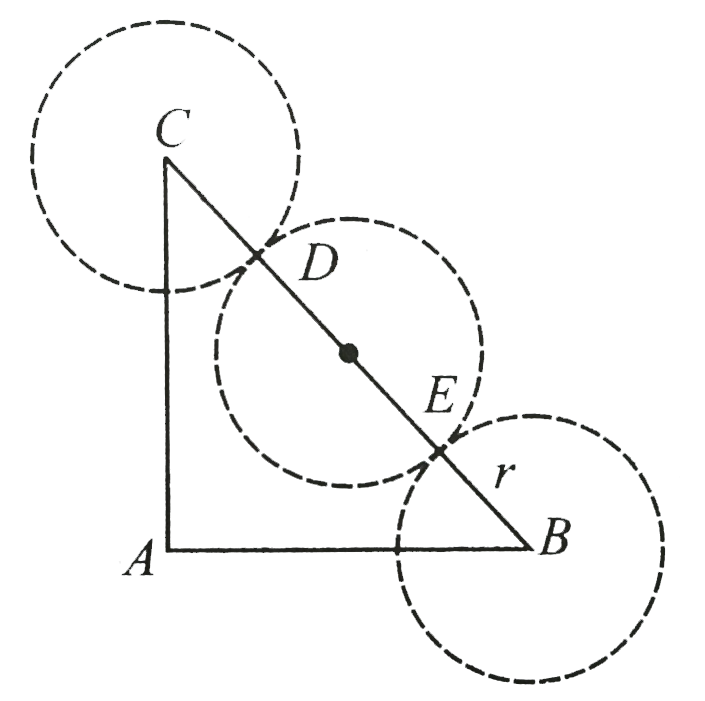
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
A2Z|Exercise Ccp, Hcp, Octahedral And Tetral Voids|17 VideosSOLID STATE
A2Z|Exercise Structure, Coordination Number, Radious Ratio Rule, Density Of Unit Cell|72 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLUTIONS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOLID STATE-Ssc, Fcc, Bcc, Nearest Neighbouring Distance, Next-Nearest Neighbouring Distance, Packing Fraction
- Copper crystallises in fcc with a cell length of 361 pm .What is the r...
Text Solution
|
- Percentage of free space in cubic close packed structure and in body c...
Text Solution
|
- Percentage of free space in cubic close packed struchure and in body c...
Text Solution
|
- In face centred cubic unit cell edge length is
Text Solution
|
- The edge length of a face centred cubic cell of an ionic substance is ...
Text Solution
|
- An elementoccurring in the bcc structure has 12.08 xx 10^(23) unit cel...
Text Solution
|
- The interionic distance for cesium chloride crystal will be
Text Solution
|
- Sodium metal crystallises in body centred cubic lattic with cell edge ...
Text Solution
|
- Lithium forms body centred cube structure .The length of the side of i...
Text Solution
|
- In a face centerd lattice of X and YX atoms are present at the corners...
Text Solution
|
- The fraction of total volume occupied by atoms in a simple cube is
Text Solution
|
- Silver metal crystallises in a cubic closed packed arrangement with ed...
Text Solution
|
- Xenon crystallises in face - centered cubic , and the edge of the unit...
Text Solution
|
- The length of the unit cell edge of a lattice metal is 350 pm .Thus vo...
Text Solution
|
- If a stand for the edge length of the cube system simple cubic , body ...
Text Solution
|
- In a face centered cubic cell , an the face contributes in the unit ce...
Text Solution
|
- The number of atom/molecules contained in one body centered cubic cell...
Text Solution
|
- Stracture of unit cell is described in the given figure packing fract...
Text Solution
|
- Potassium crystallizes with a
Text Solution
|
- The packing fraction of the element that crystallizes in simple cubic ...
Text Solution
|